Study Design
A FOAM TO ADDRESS
THE DIVERSE NEEDS OF
SEBORRHEIC DERMATITIS
PATIENTS


FIRST-IN-CLASS FOR SEBORRHEIC DERMATITIS2
STRATUM and Trial 203 pivotal study designs1,2:
- Two multicenter, randomized, double-blind,
vehicle-controlled studies - 683 participants with moderate to severe
seborrheic dermatitis- STRATUM: ZORYVE foam = 304, vehicle = 153
- Trial 203: ZORYVE foam = 154, vehicle = 72
- Once daily for 8 weeks
- Diagnosis of moderate (IGA = 3) or severe (IGA = 4) seborrheic dermatitis1
- Age ≥9 years in STRATUM and ≥18 years in Trial 2031
- ≤20% BSA2
BSA = Body Surface Area.
No concomitant therapies or other moisturizers/emollients were allowed on treated areas2
Primary Endpoint
Key Secondary Endpoints In STRATUM
IGA Success at Week 8
Achievement of an IGA score of Clear (0) or Almost Clear (1) and a ≥2-grade improvement from baseline
IGA = Investigator Global Assessment.
IGA 0 at Week 8
Achievement of completely Clear skin (IGA = 0)3
WI-NRS Success at Weeks 2, 4, and 8
Achievement of a ≥4-point improvement for patients with a baseline score of ≥43
IGA = Investigator Global Assessment; WI-NRS = Worst-Itch Numeric Rating Scale.
73% of patients had involvement in multiple areas in STRATUM2
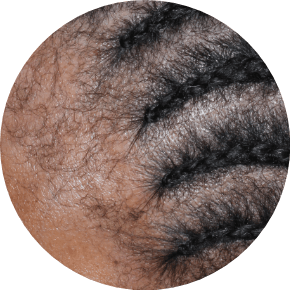
SCALP
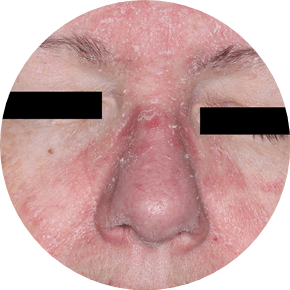
FACIAL
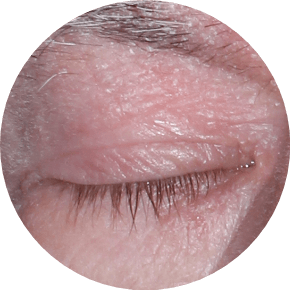
EYELIDS
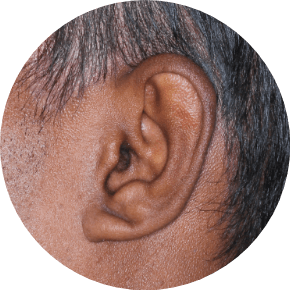
EARS
TRUNK
Actual clinical trial patients

