Before & After
Consistent RESULTS WHEREVER PATIENTS
NEED IT
ZORYVE is for topical use only and not for ophthalmic, oral, or intravaginal use1,2


ZORYVE CREAM. ONCE A DAY. ANYWHERE.
Review clinical trial photos of patients with plaque psoriasis treated with ZORYVE cream
ZORYVE CREAM. ONCE A DAY. ANYWHERE.
Review clinical trial photos of patients with plaque psoriasis treated with ZORYVE cream
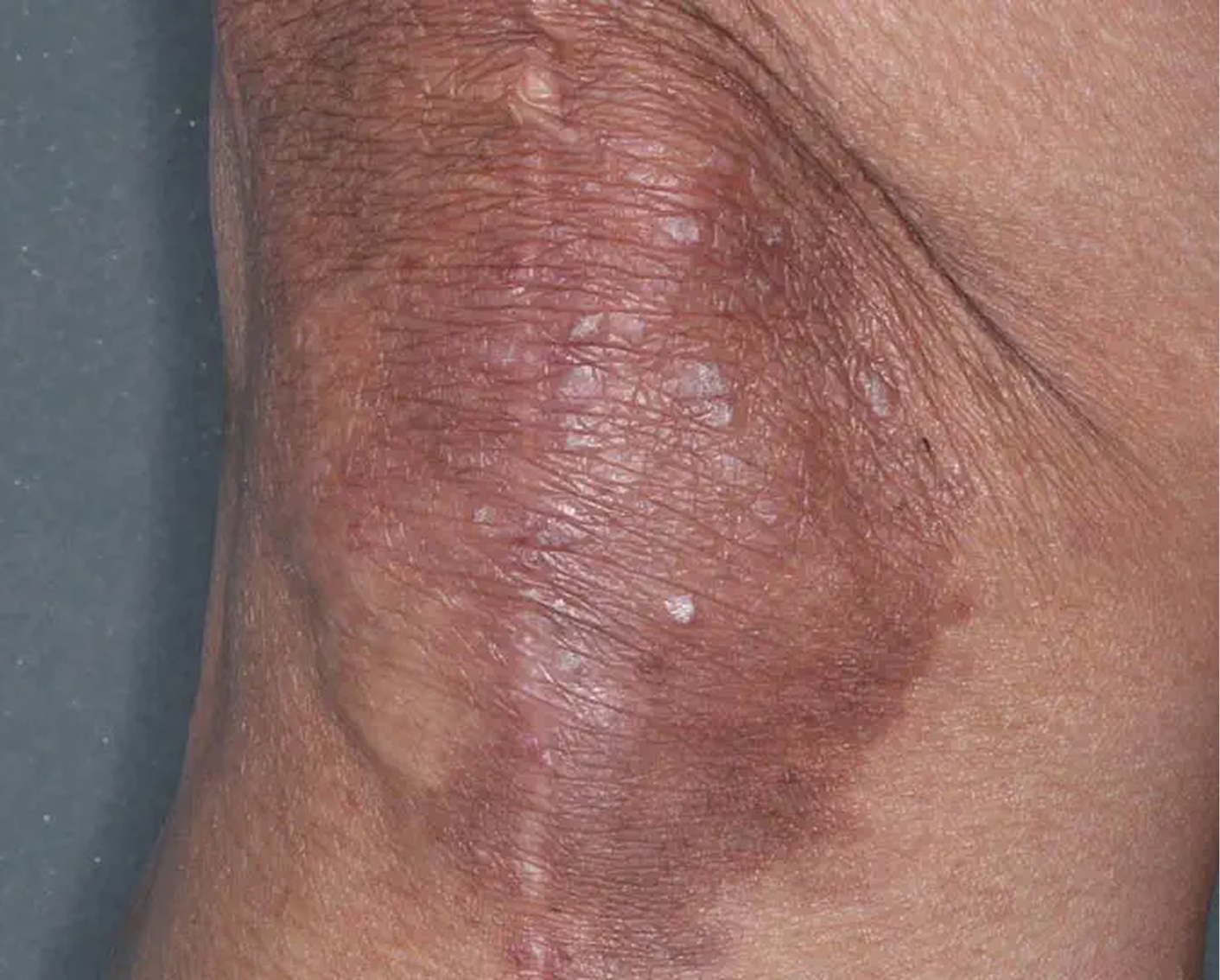
Knee3
AGE 59, FEMALE
Actual clinical trial patient
Baseline: IGA = 3
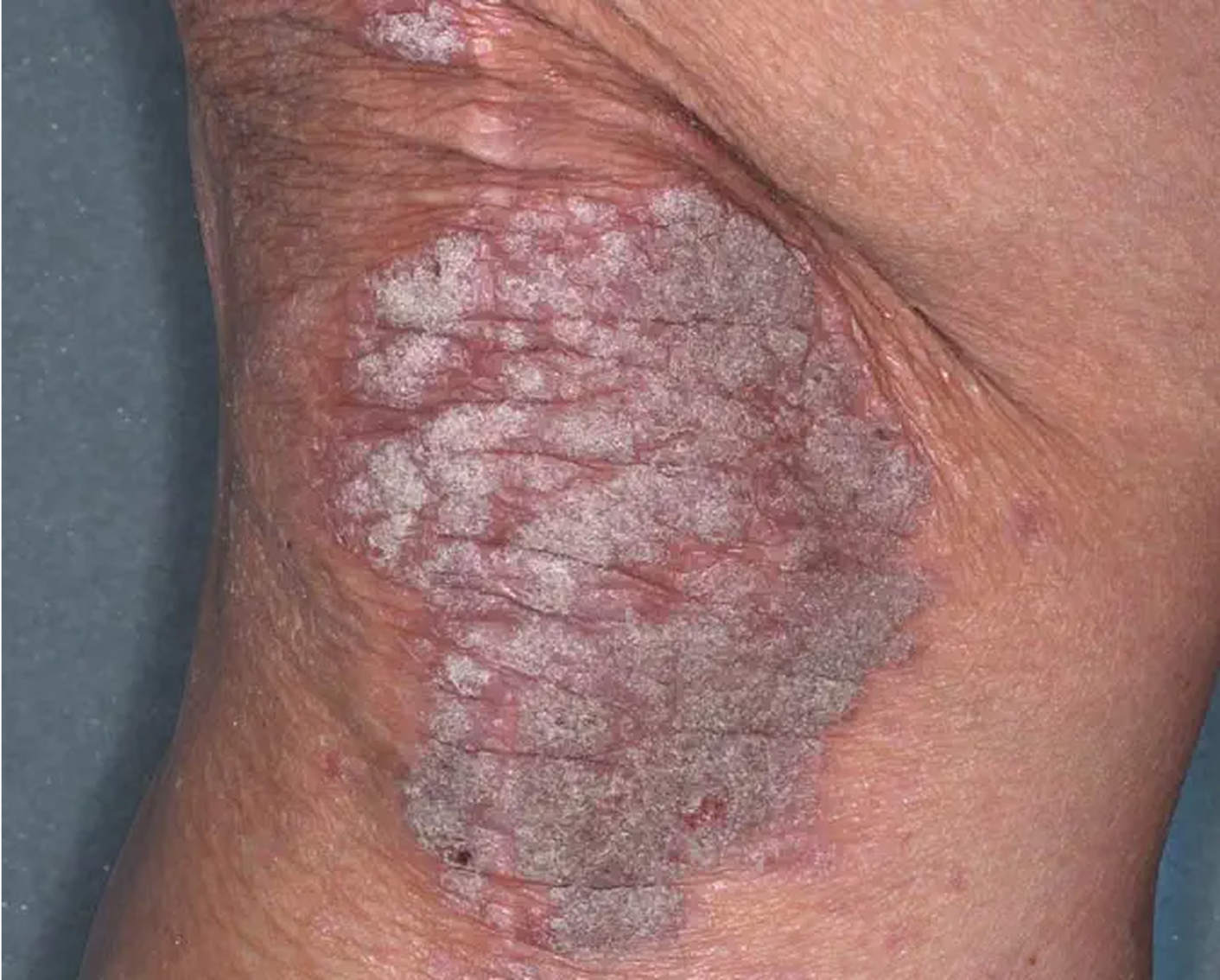
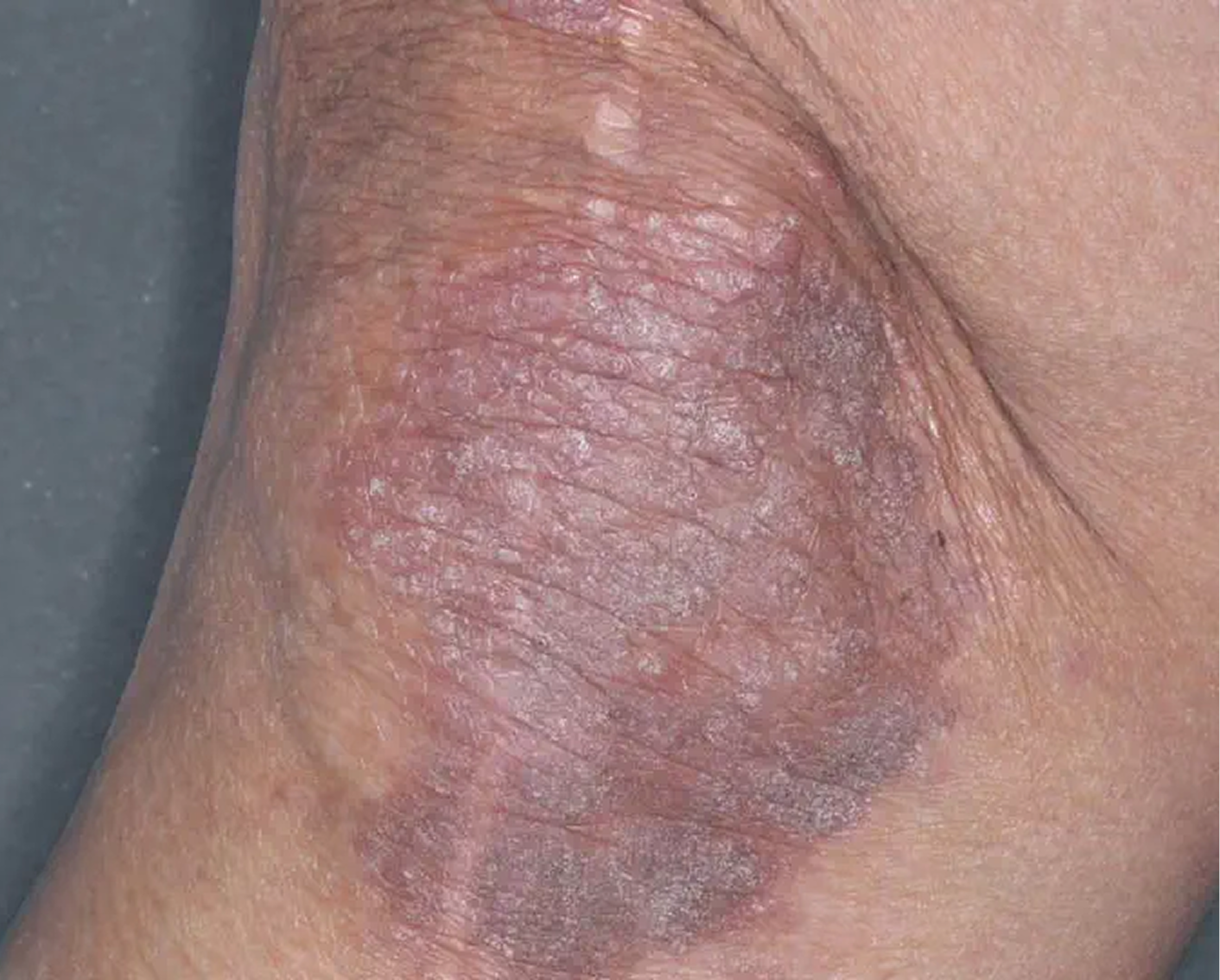
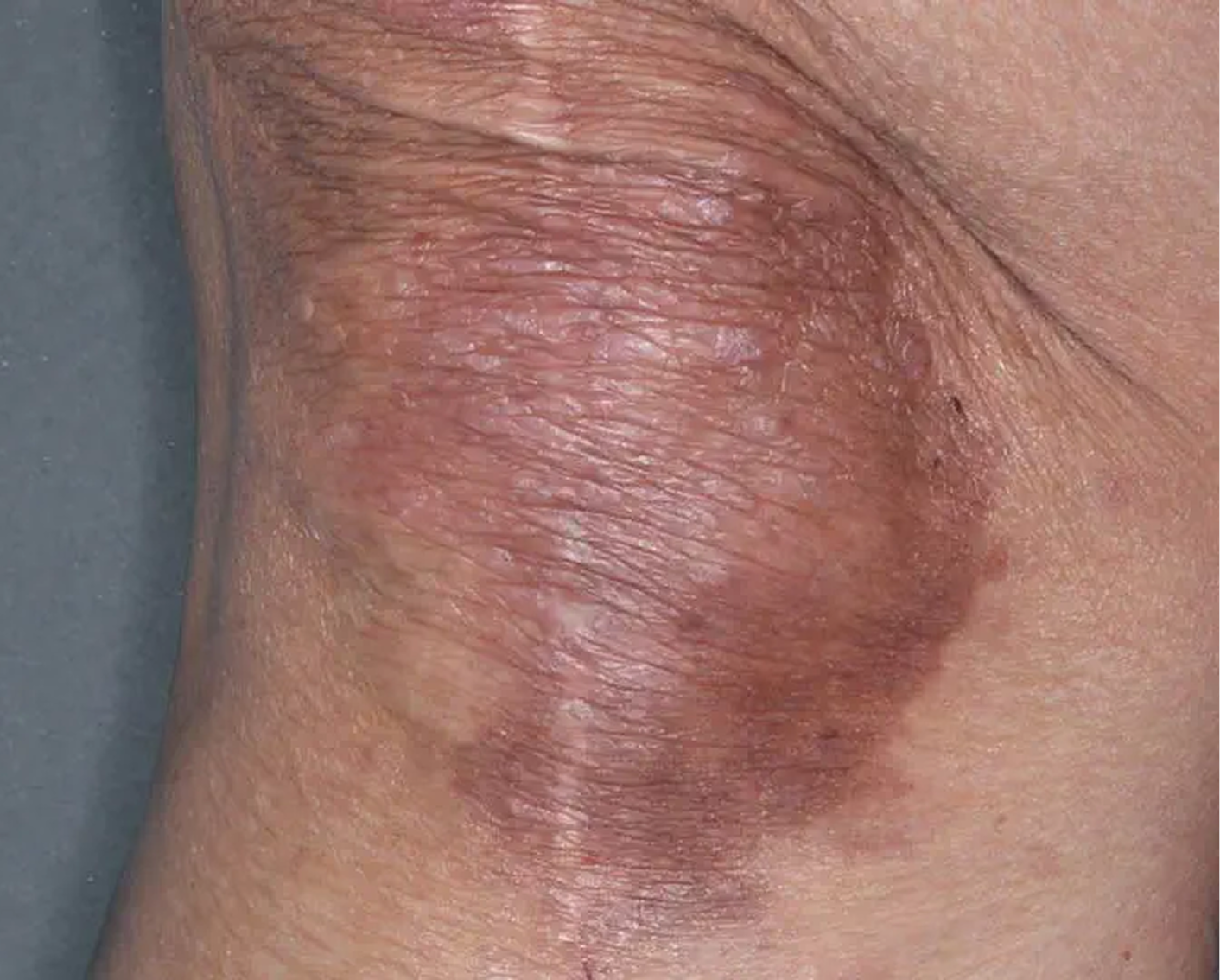
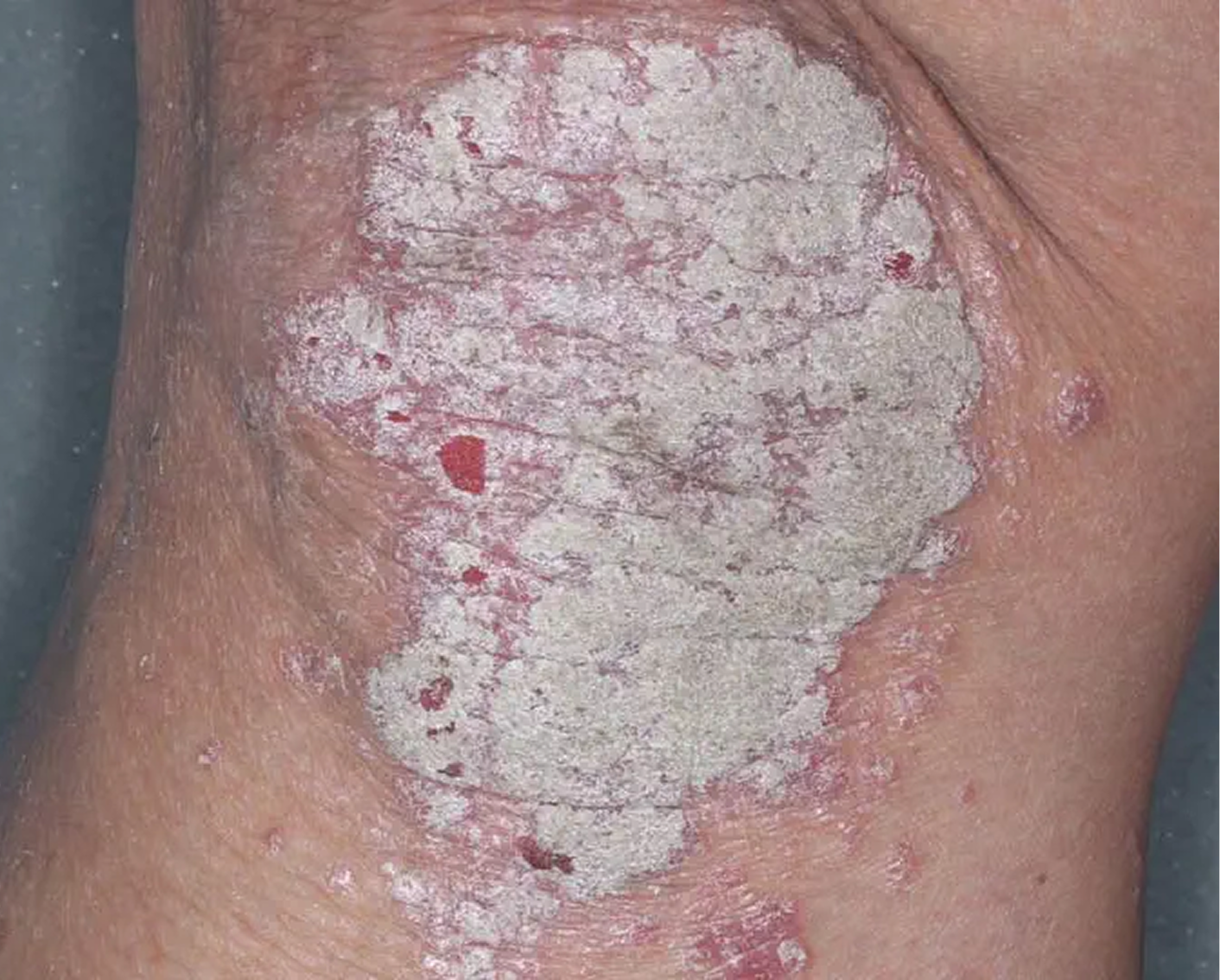
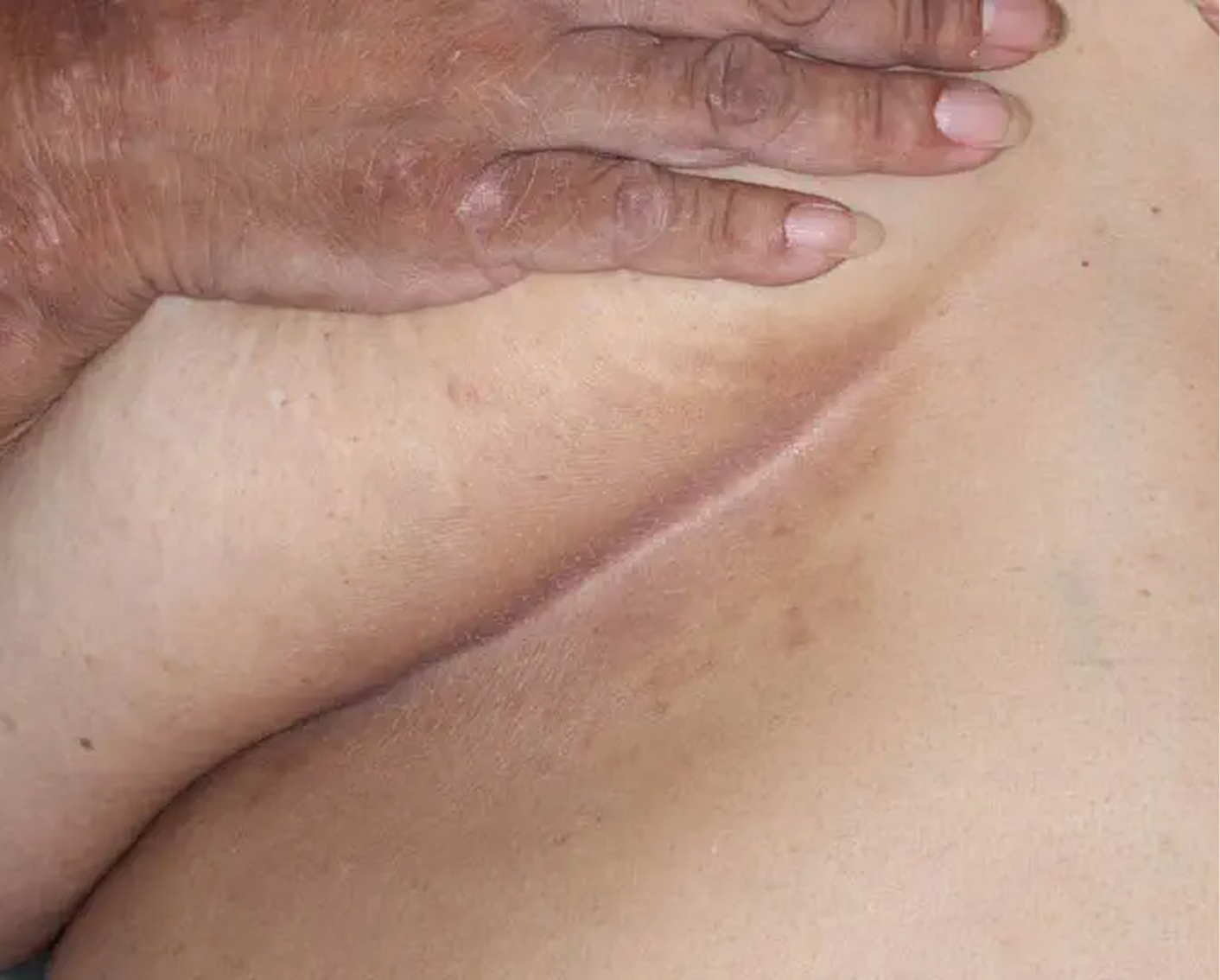
Inframammary Crease3
AGE 59, FEMALE
Actual clinical trial patient
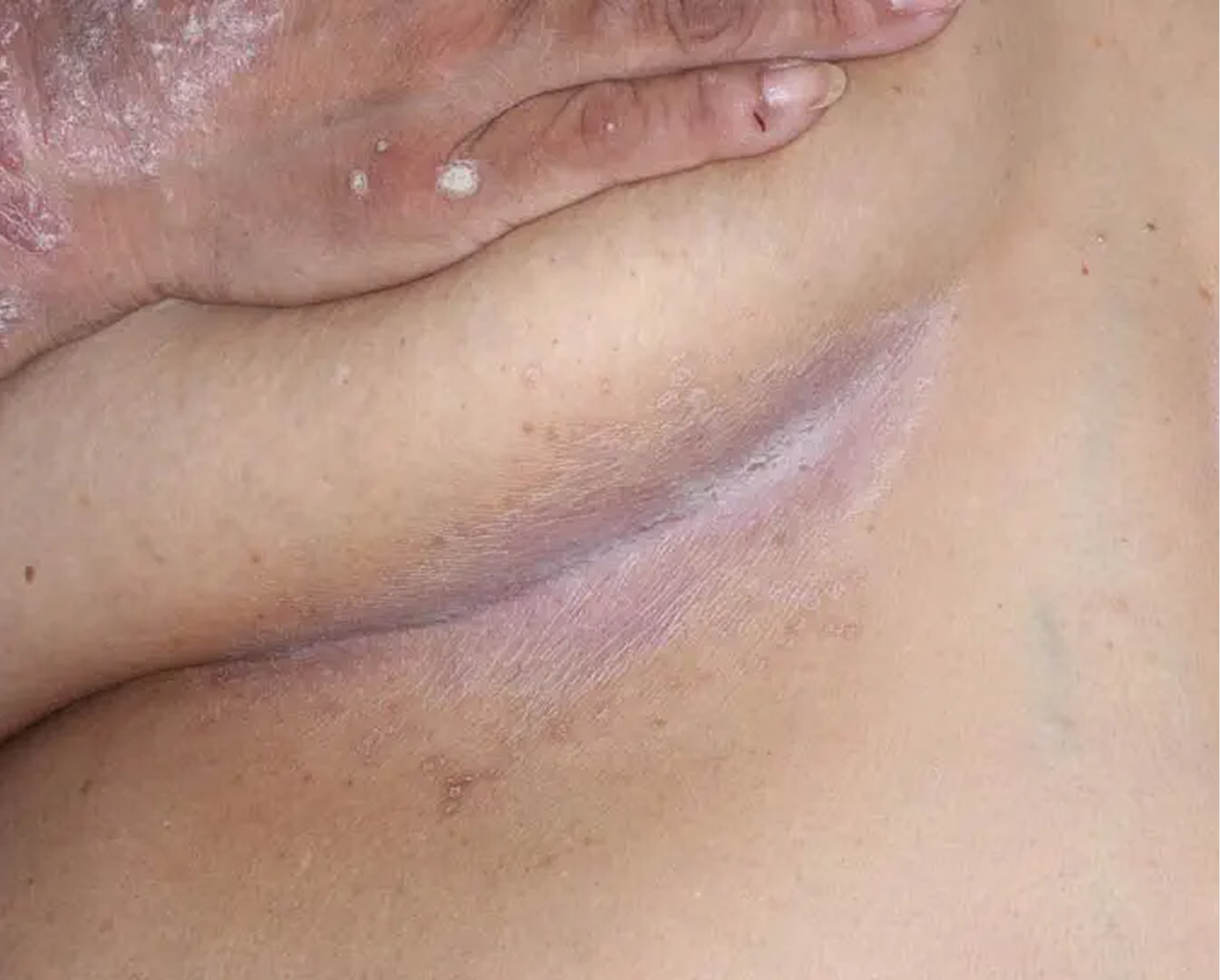
Baseline: I-IGA = 2
POWERFUL CLEARANCE IN HARD-TO-TREAT AREAS2
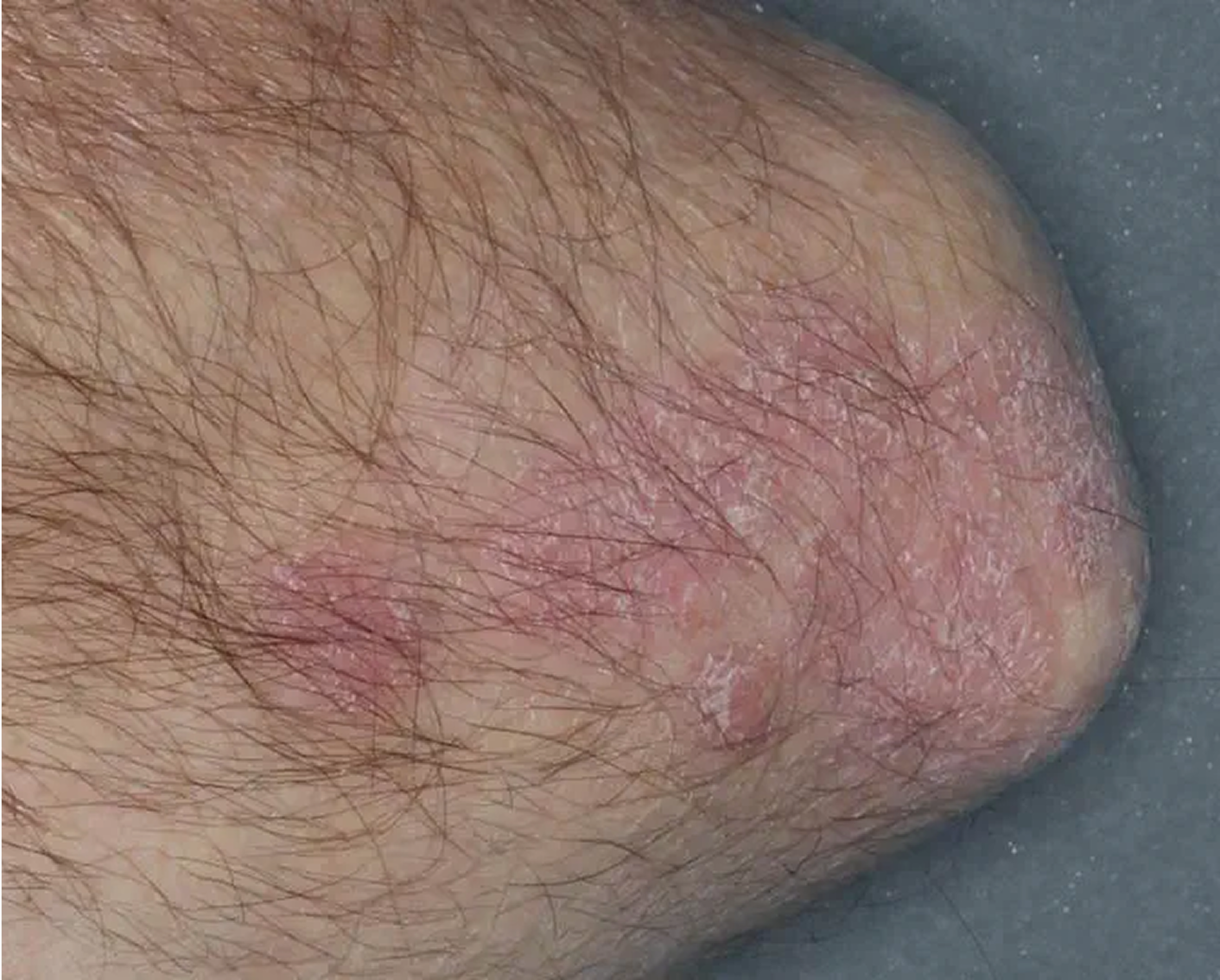
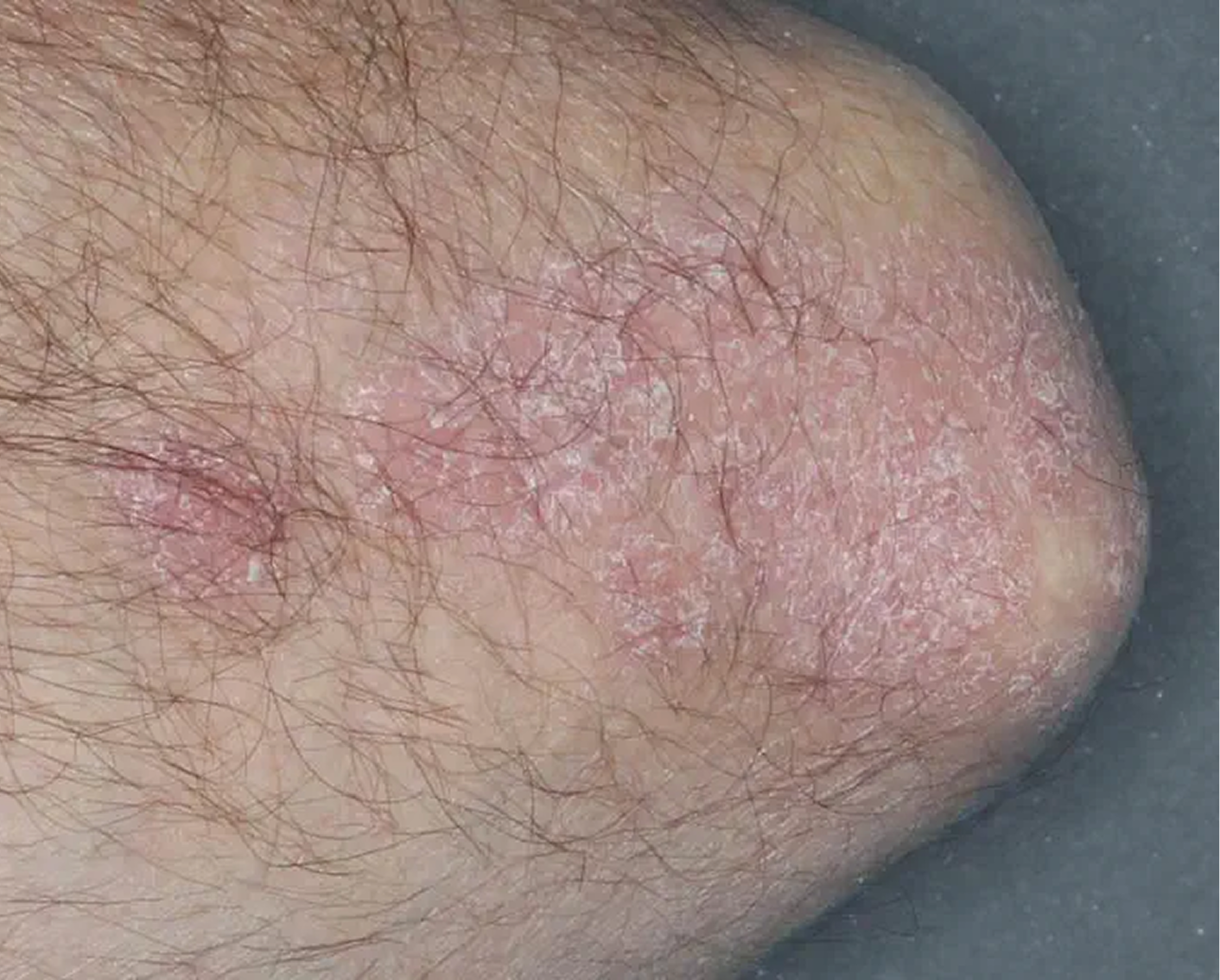
Elbow3
AGE 58, MALE
Actual clinical trial patient
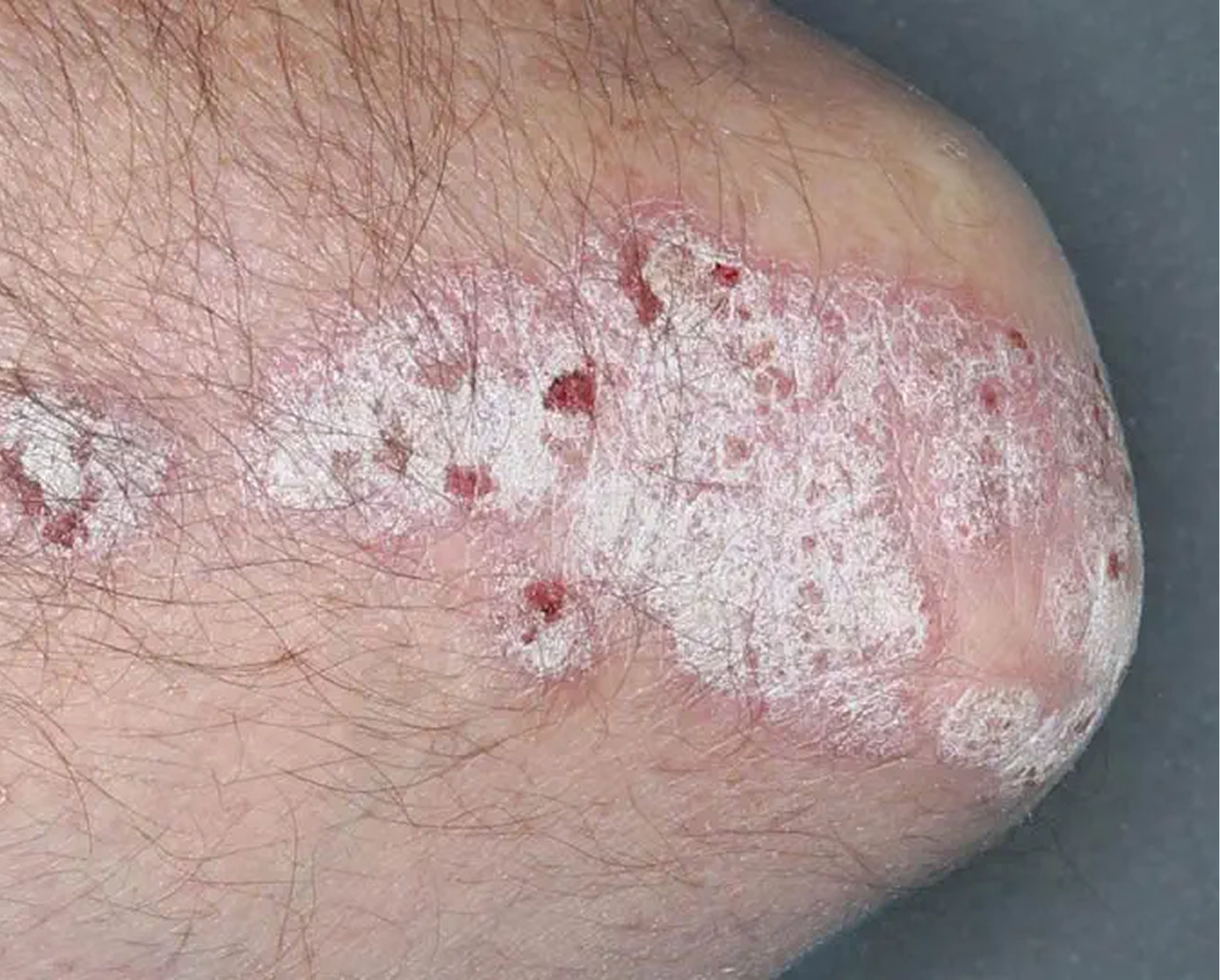
Baseline: IGA = 4
Heel3
AGE 53, FEMALE
Actual clinical trial patient
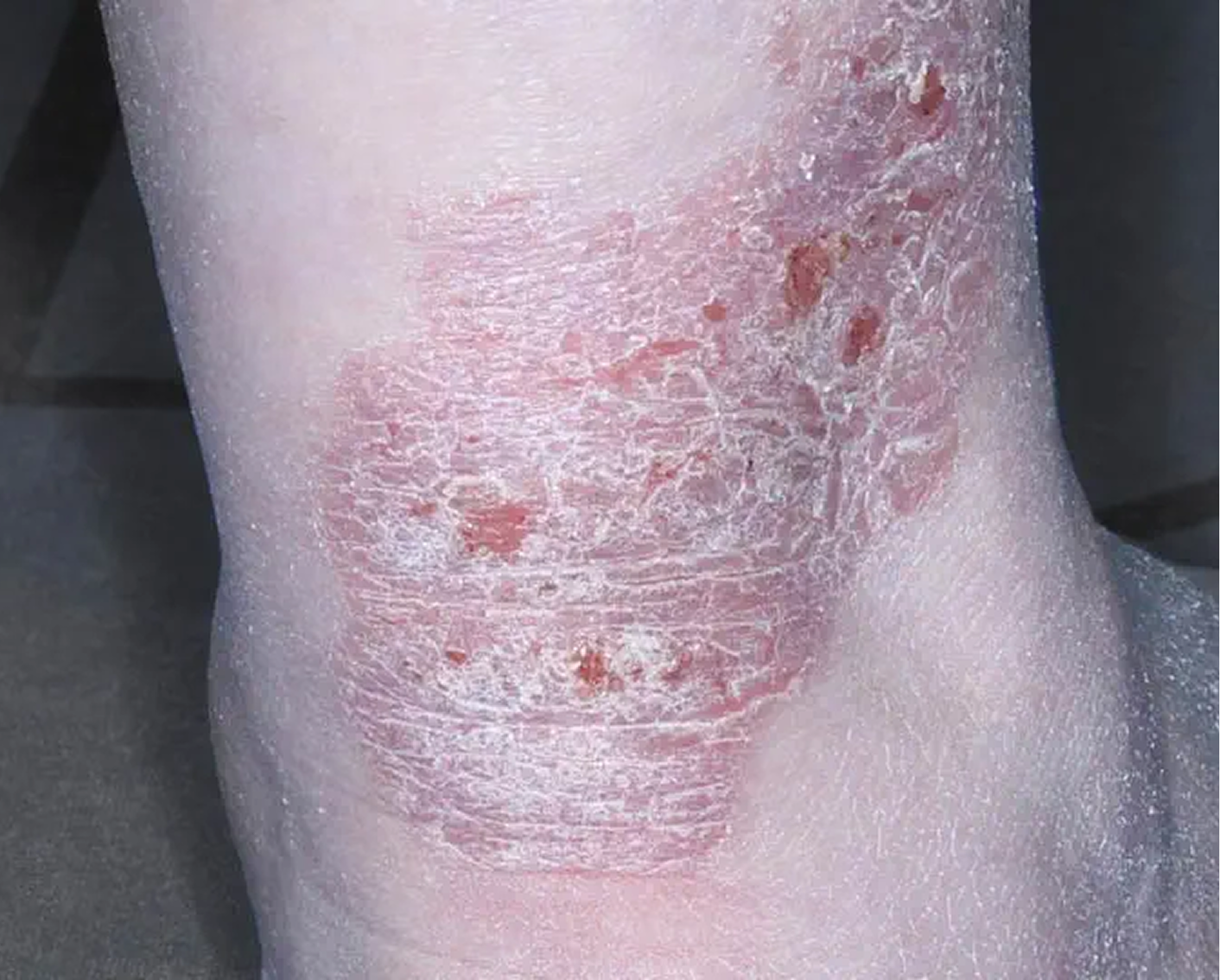
Baseline: IGA = 3
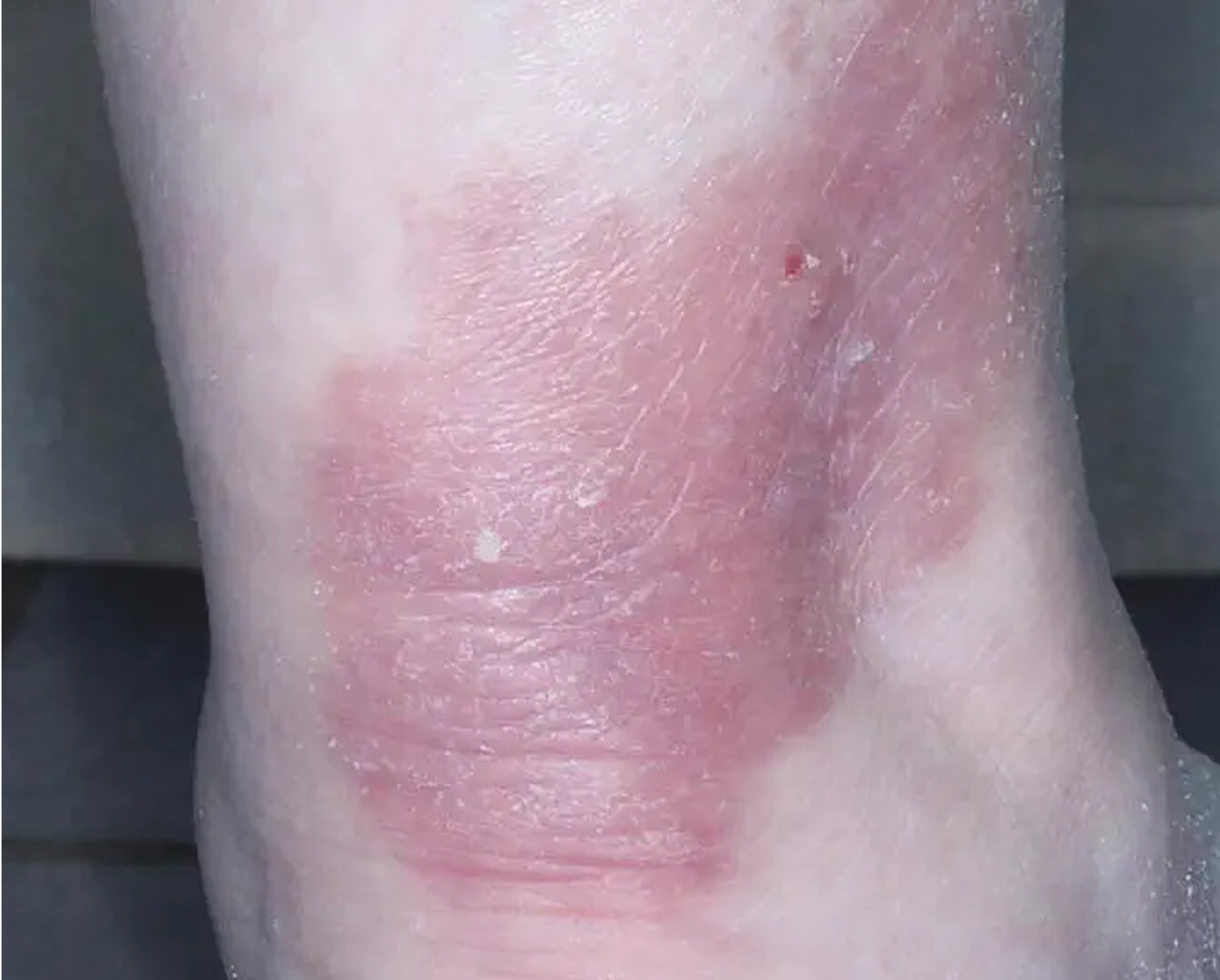
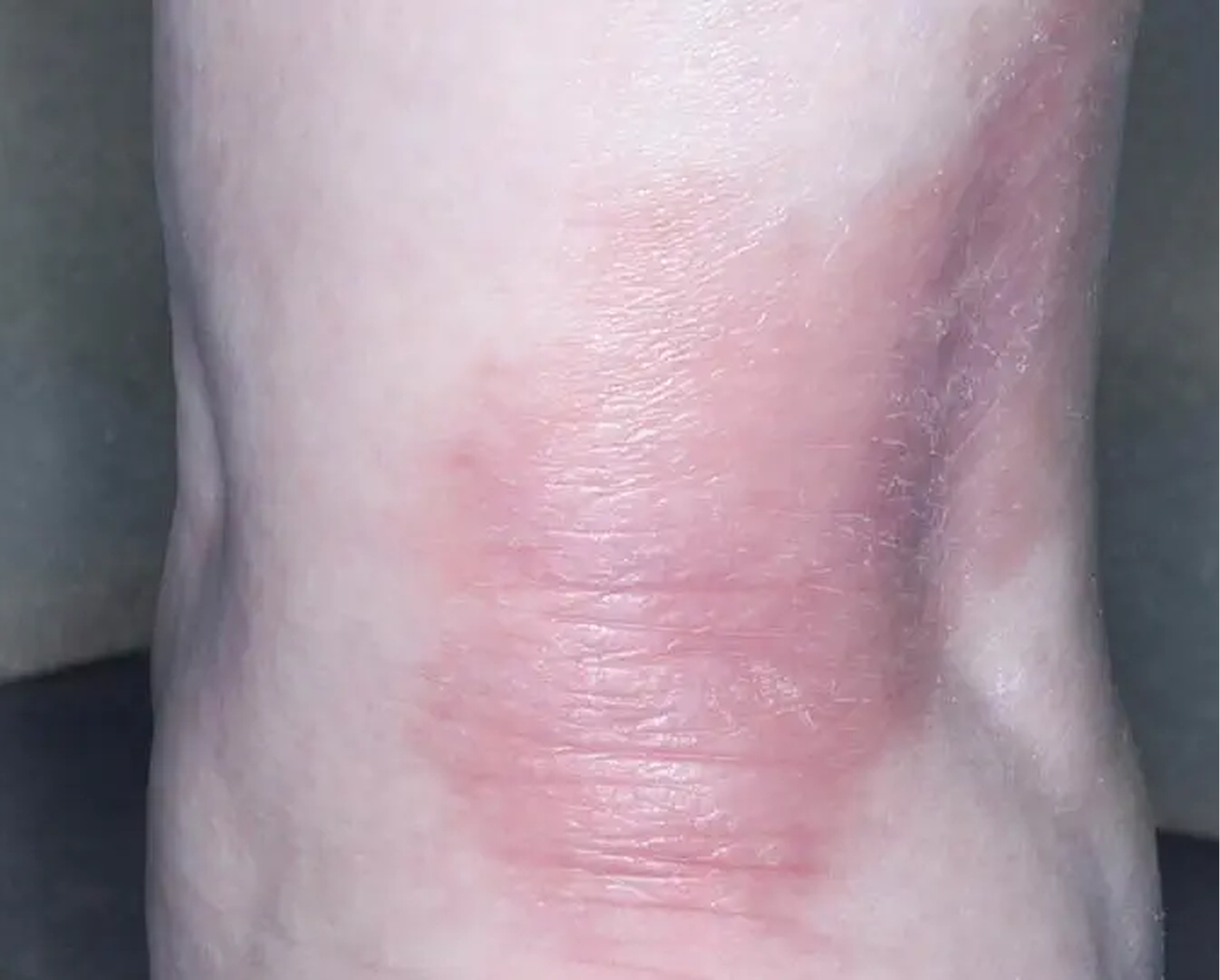
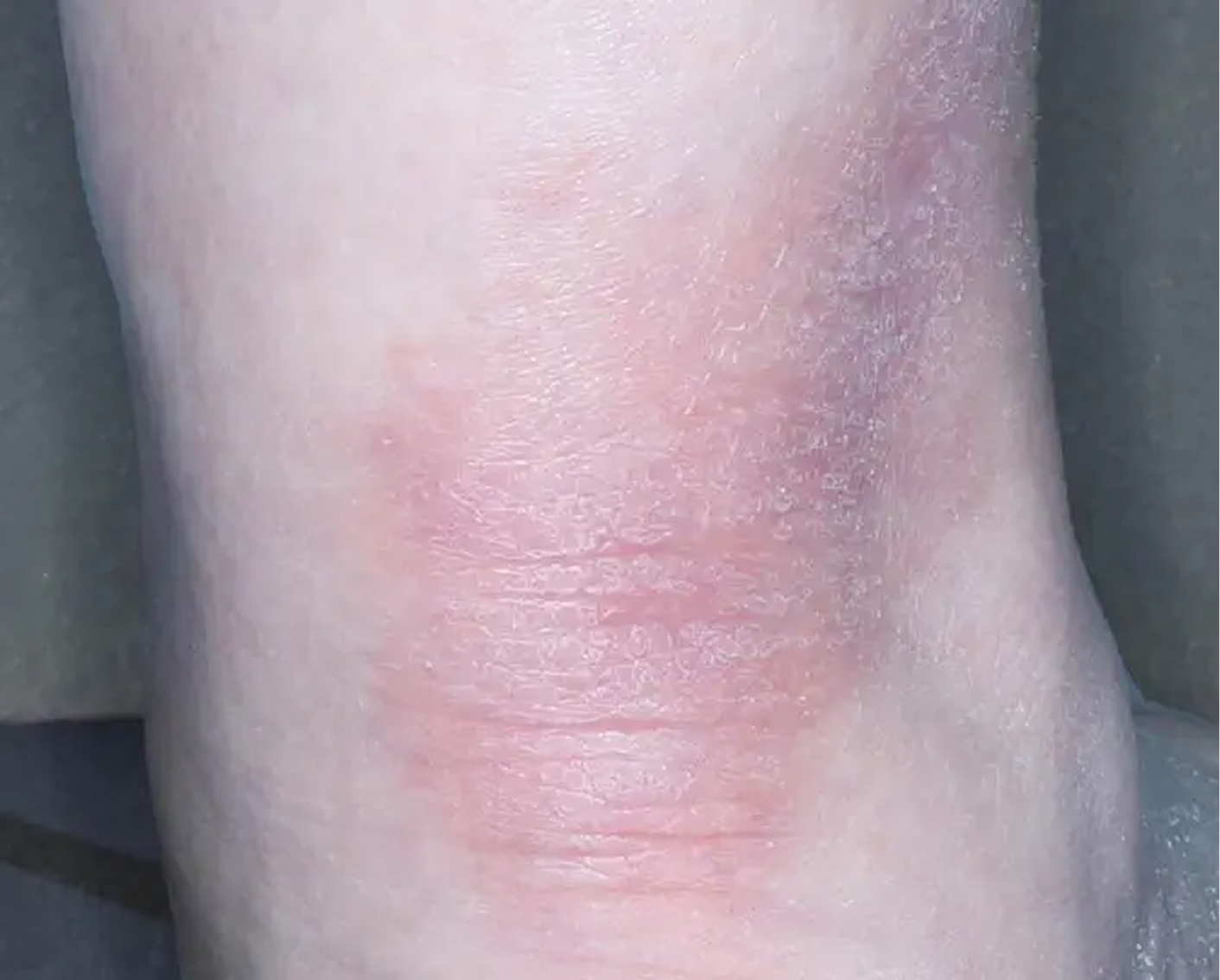
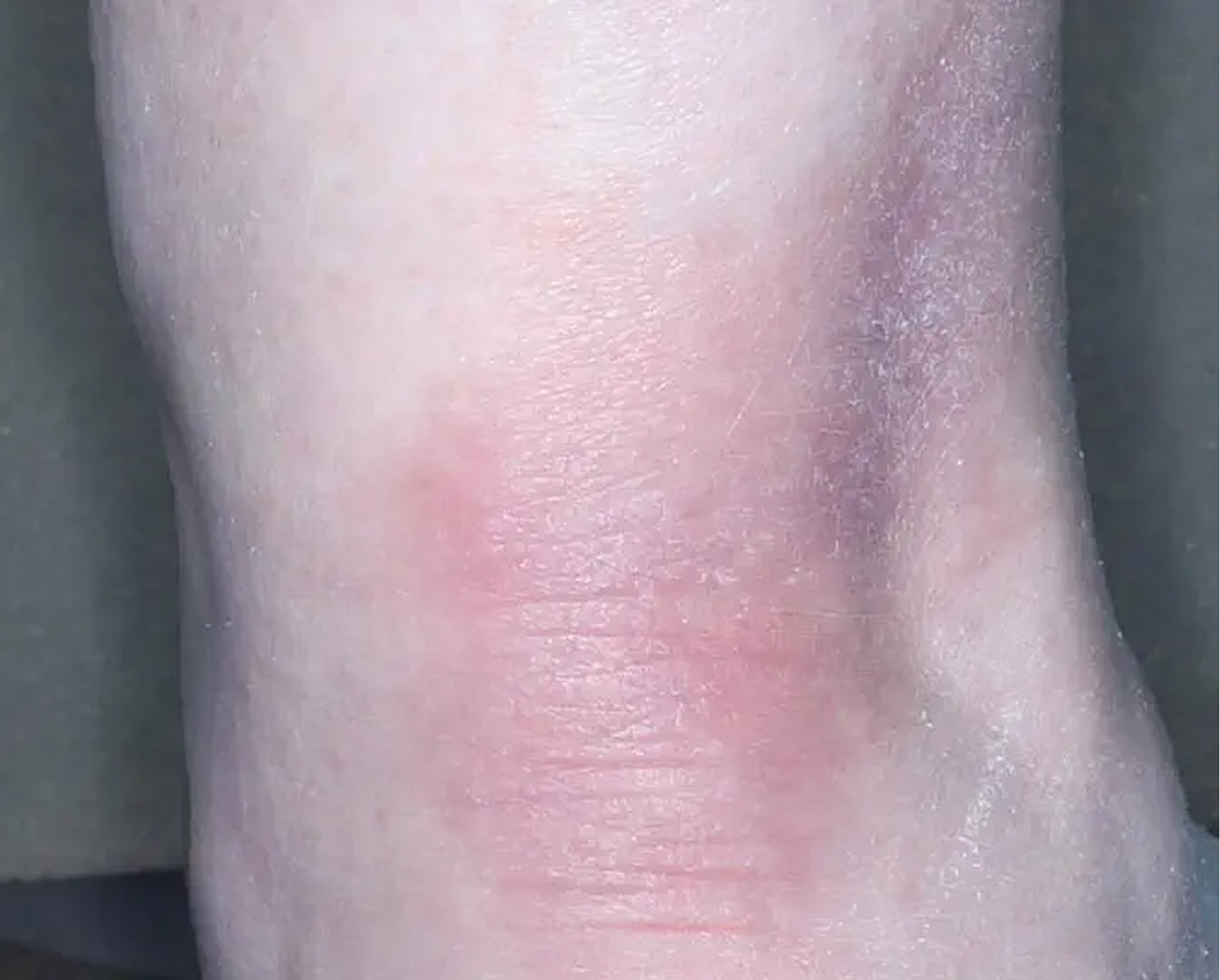
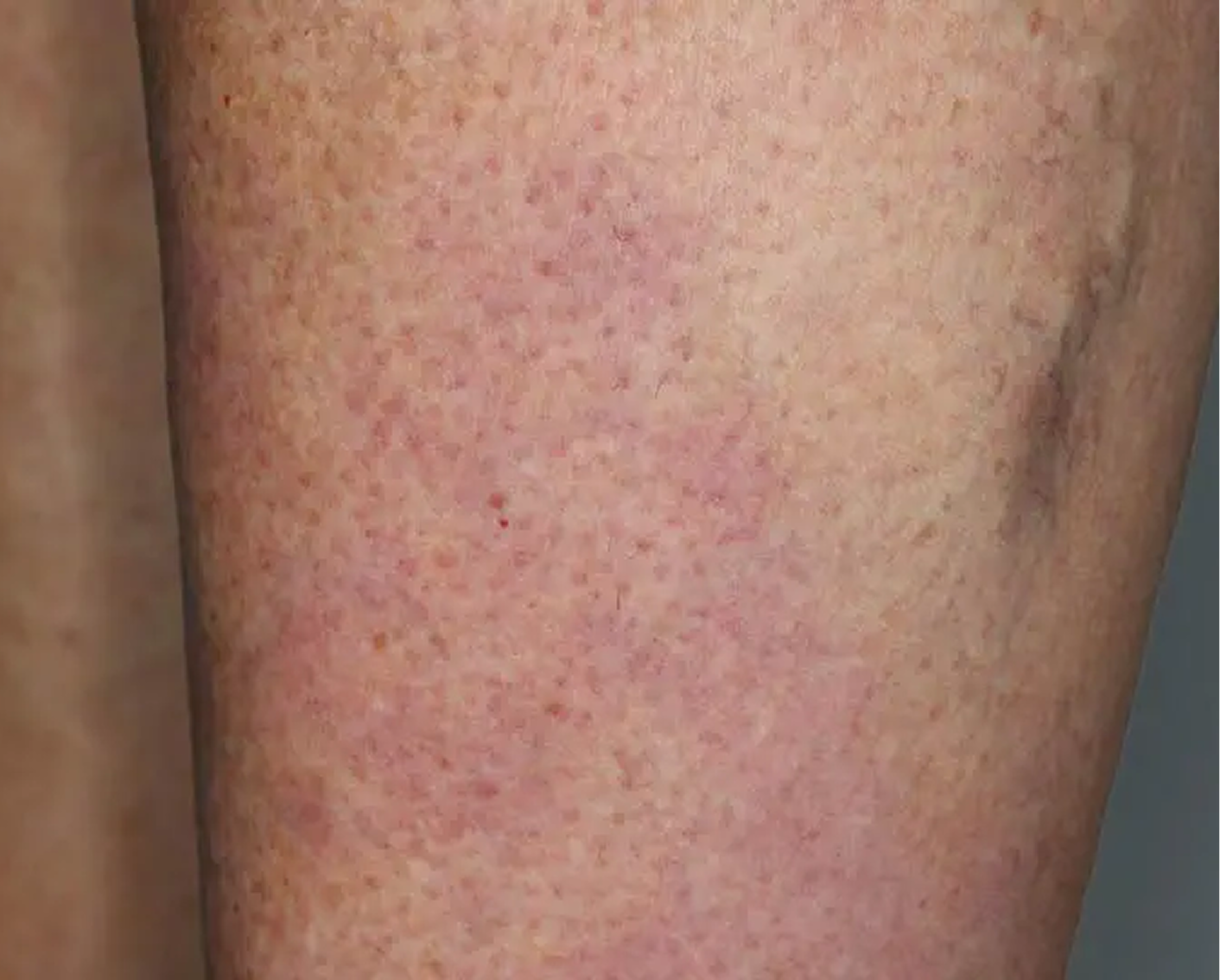
Shin3
AGE 61, FEMALE
Actual clinical trial patient
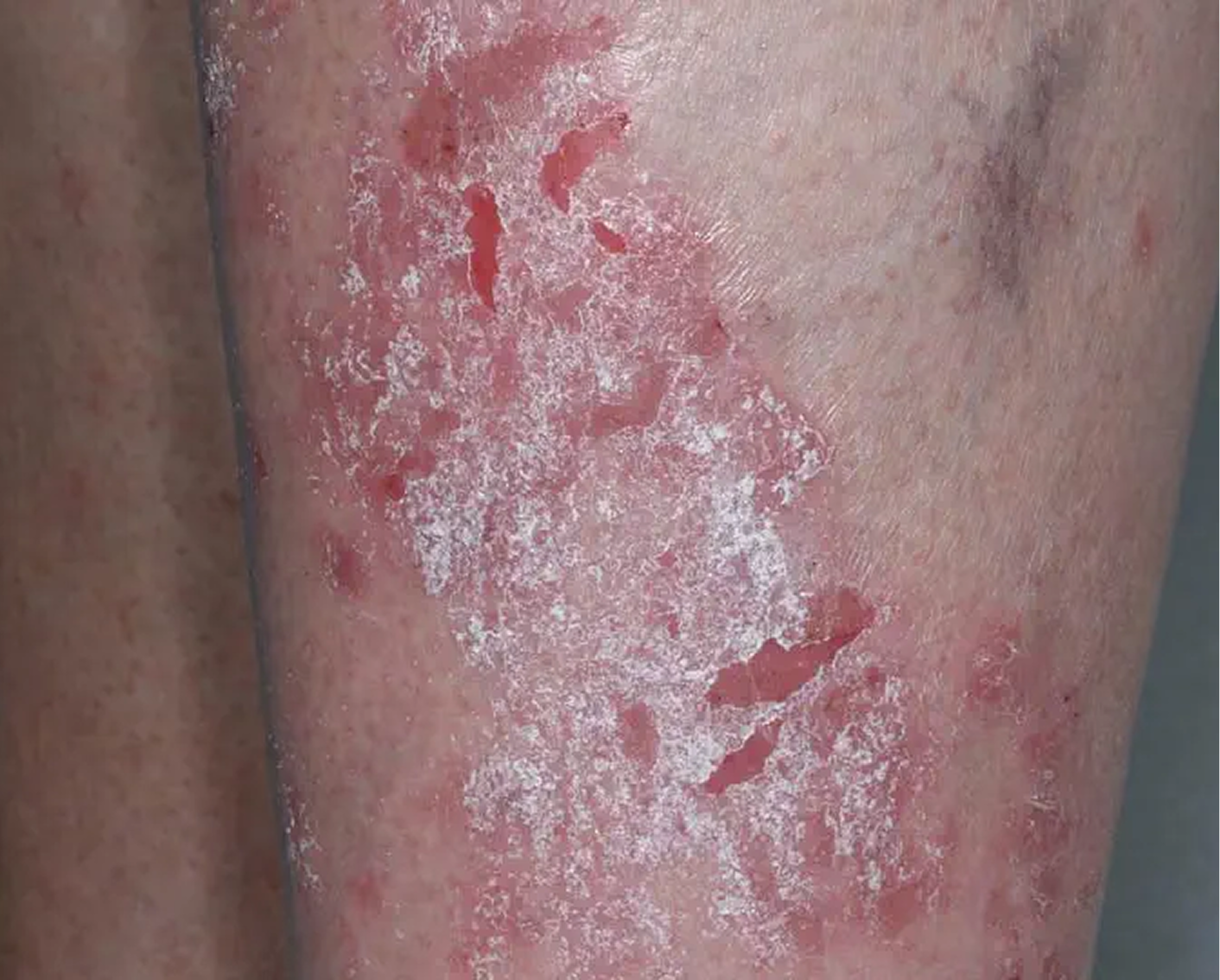
Baseline: IGA = 3
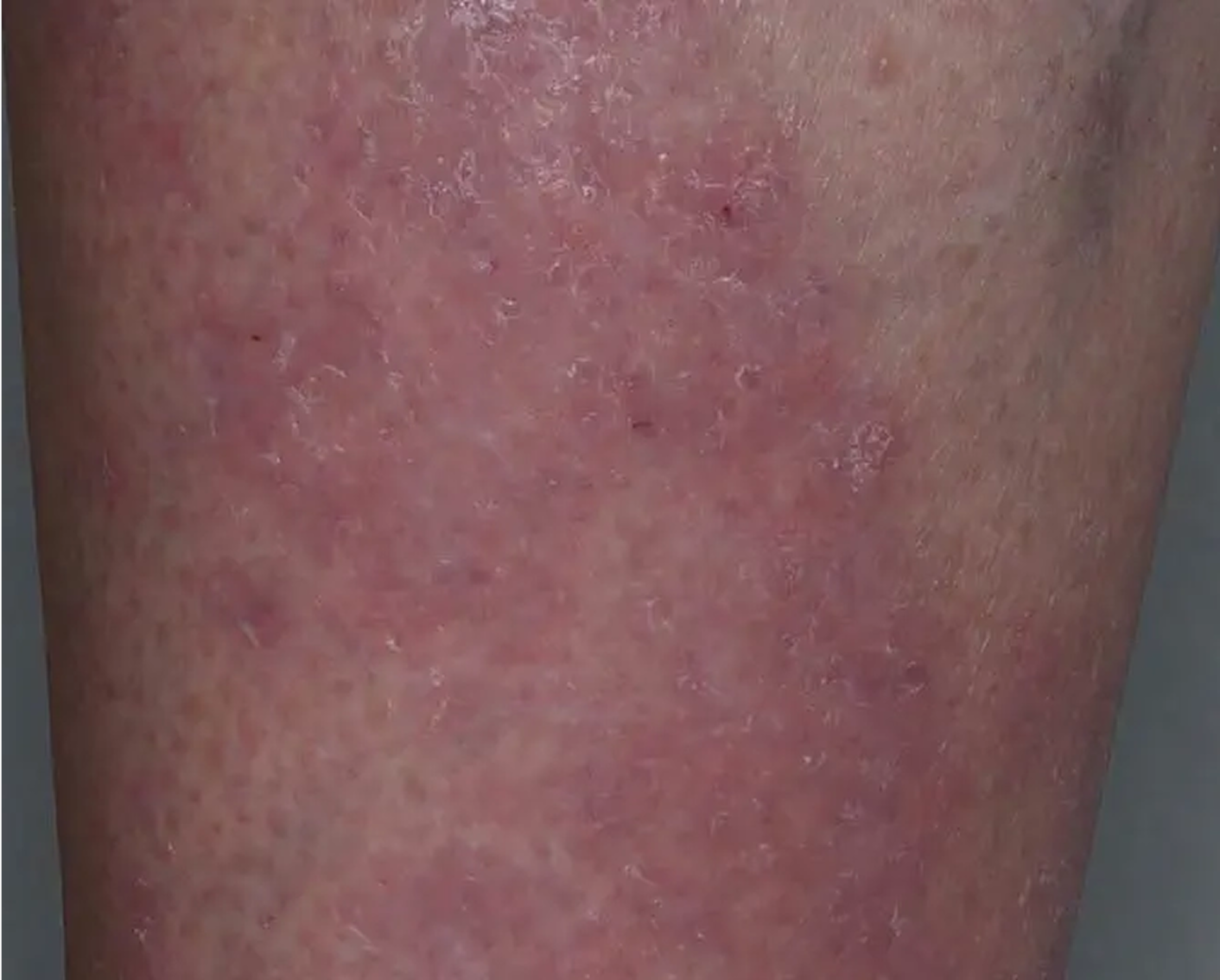
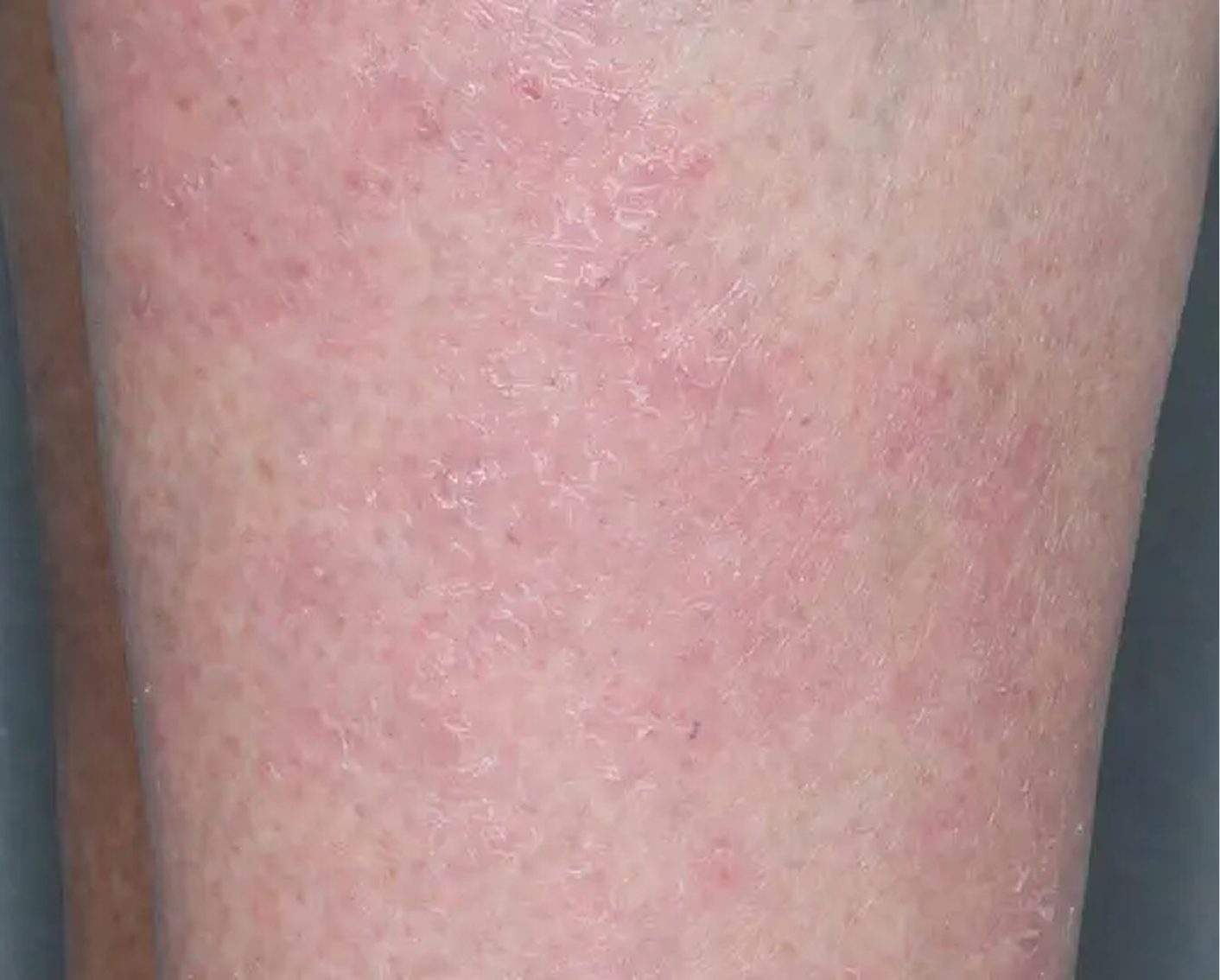
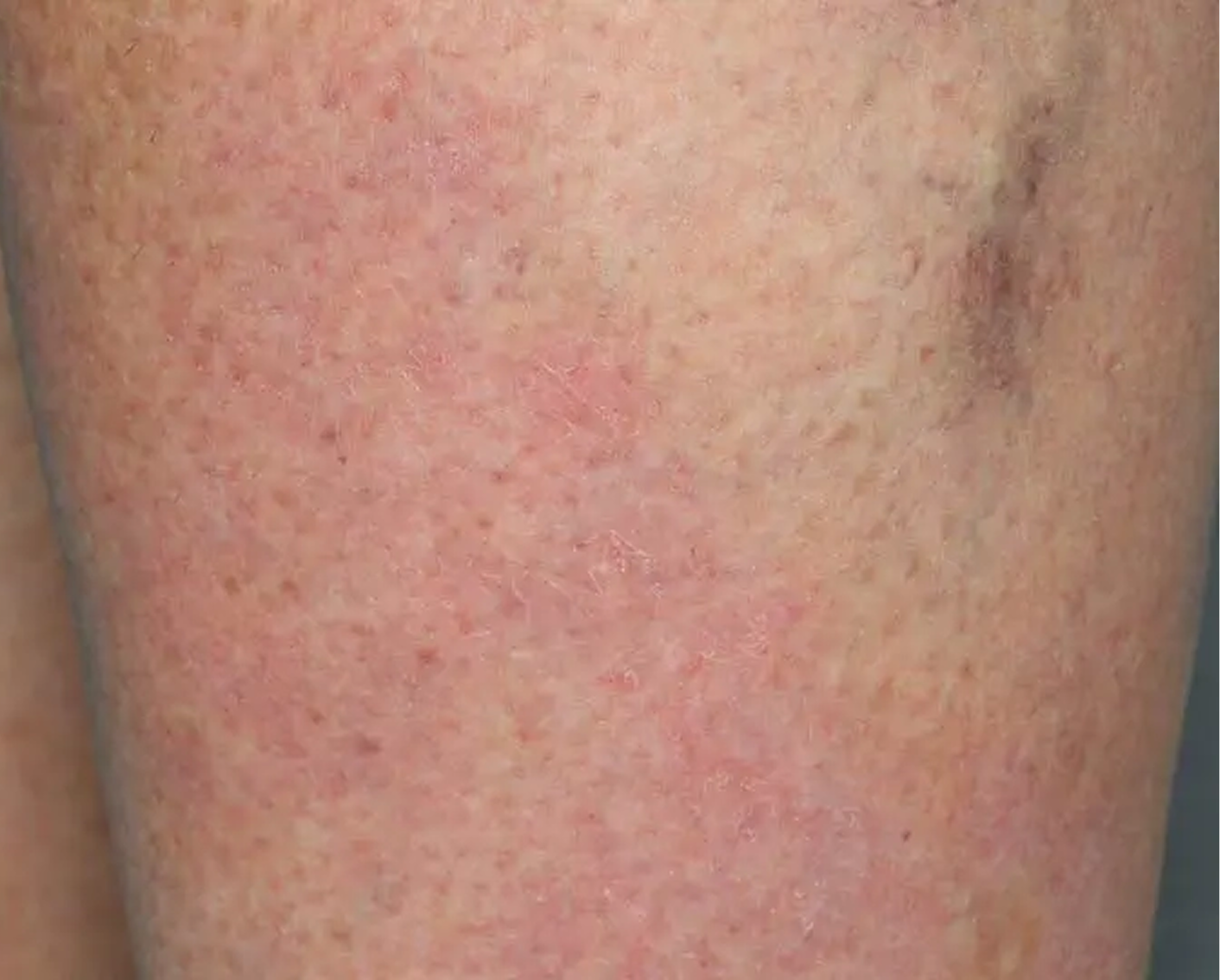
Secondary Endpoint
6x
as many patients with knee or elbow involvement achieved IGA of Clear (0) or Almost
Clear (1) with ZORYVE cream (49%, n=446) at Week 8 vs vehicle (8%, n=226)4
CLEARANCE ANYWHERE PATIENTS NEED IT2
Face & Neck3
AGE 54, MALE
Actual clinical trial patient
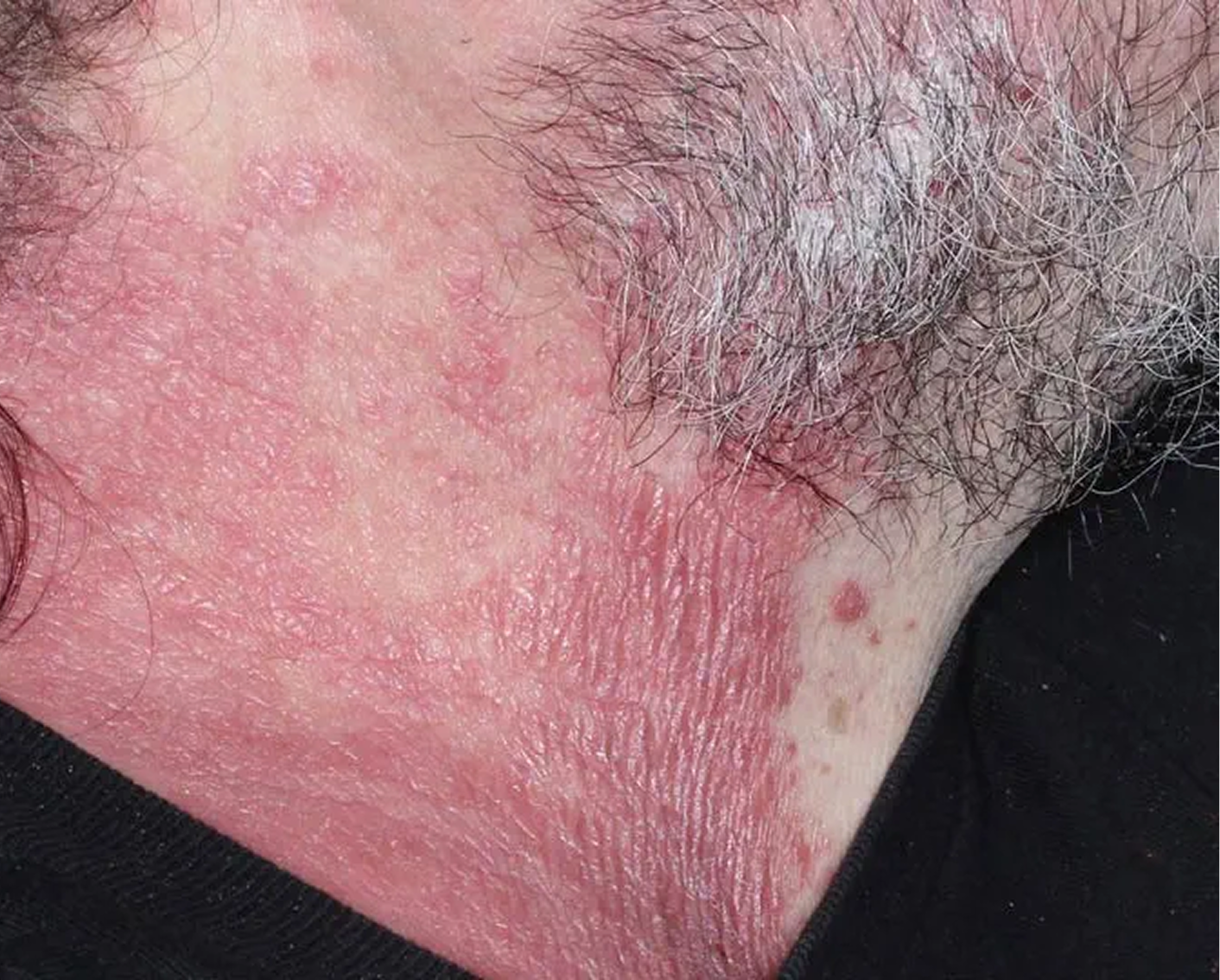
Baseline: IGA = 3
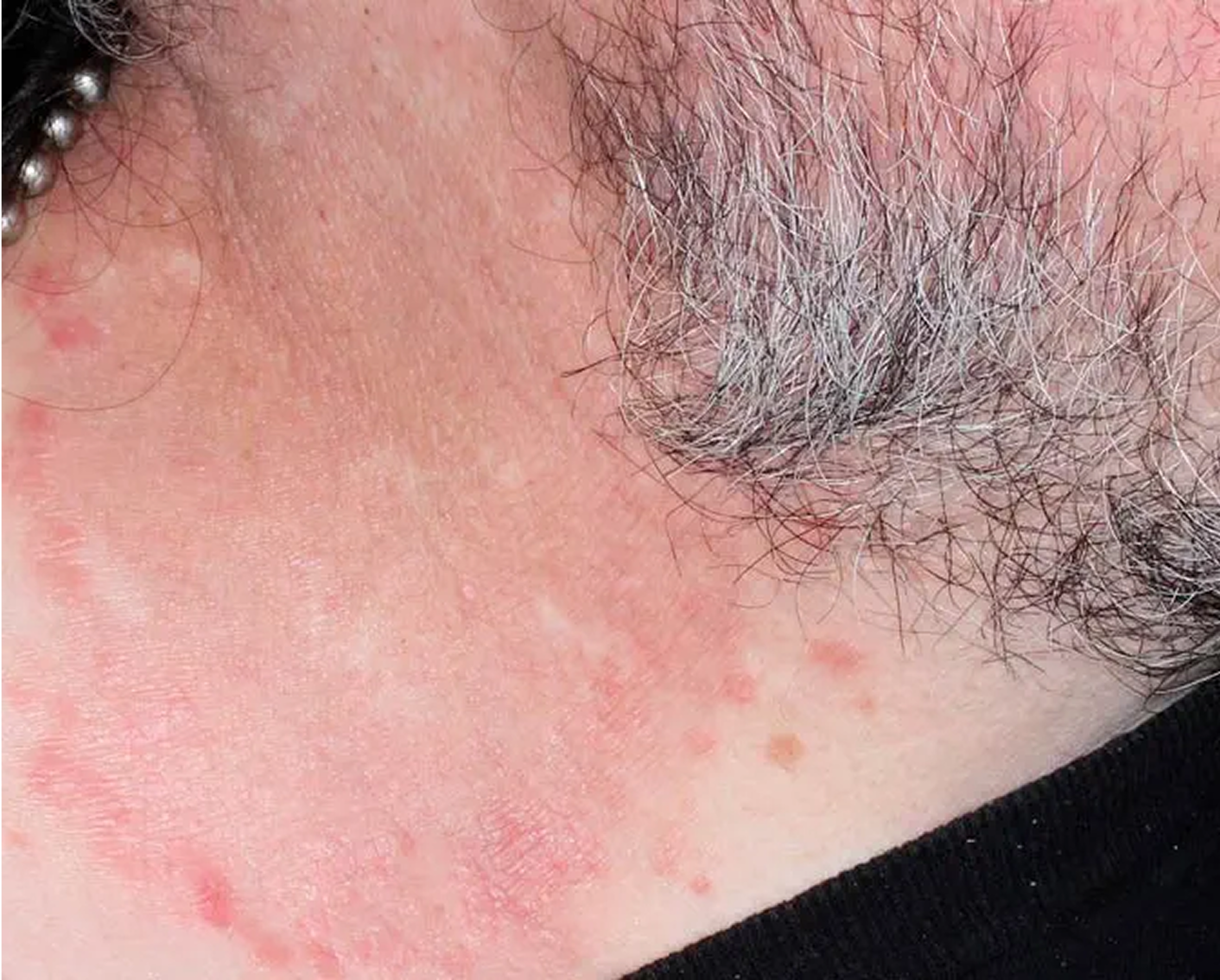
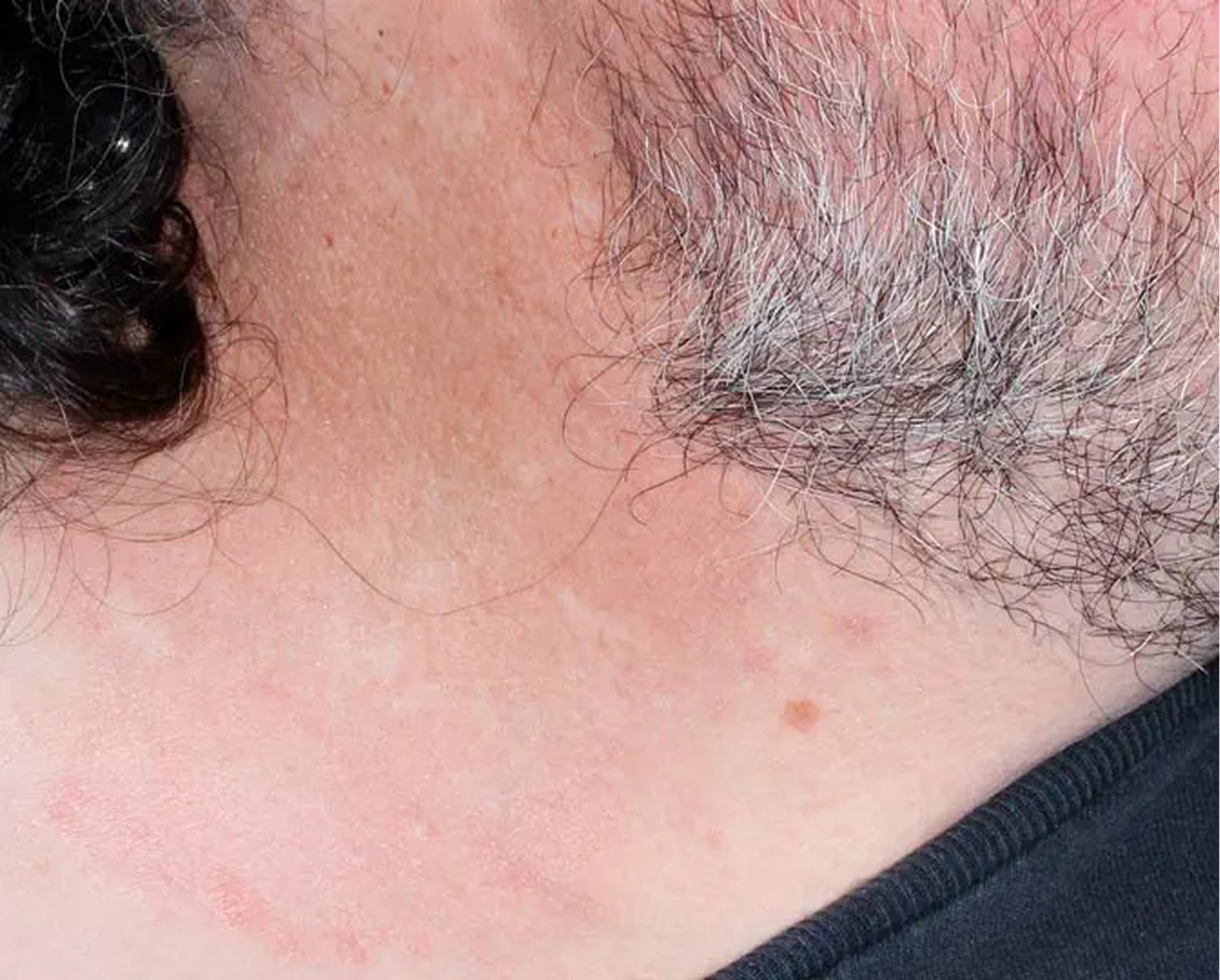
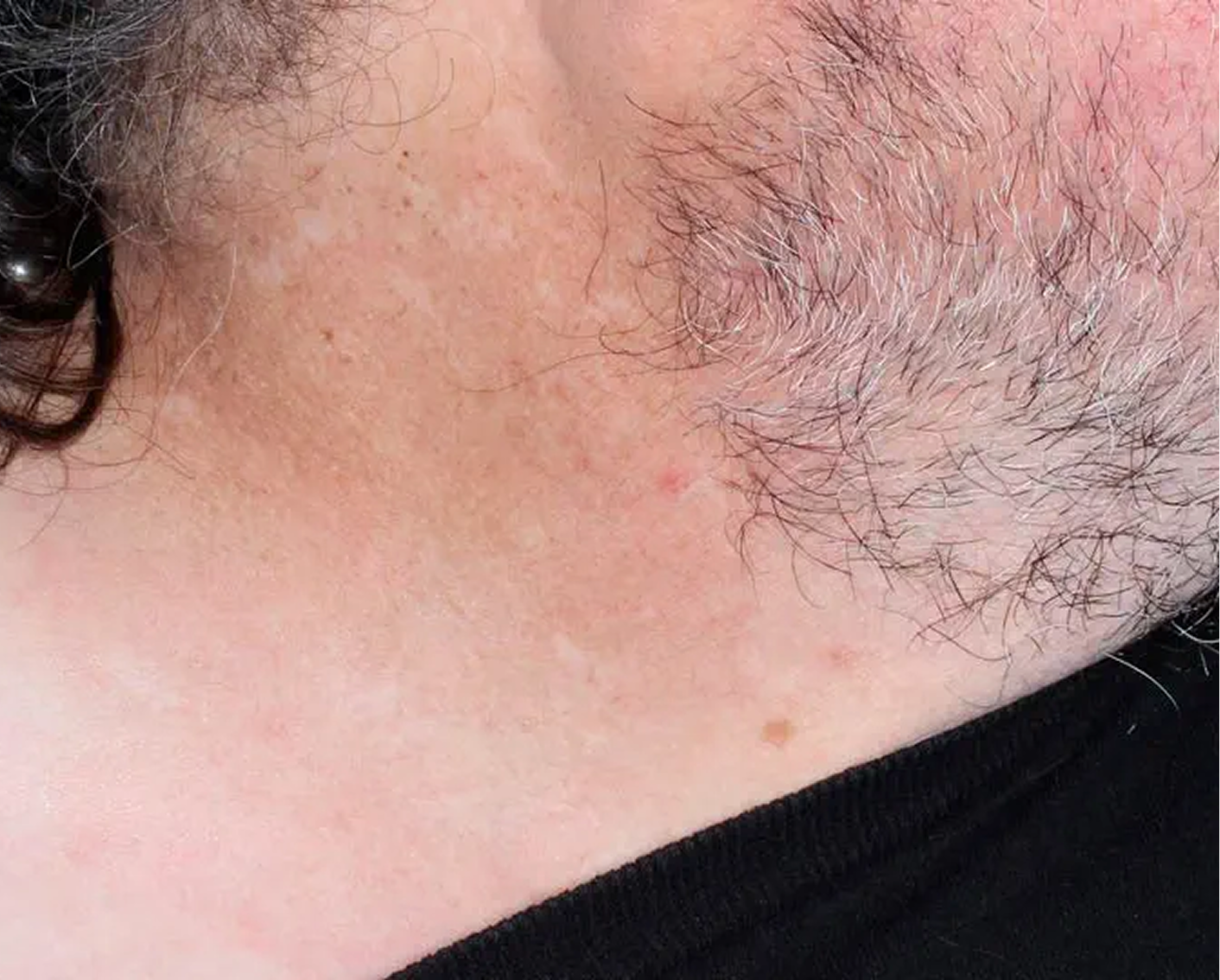
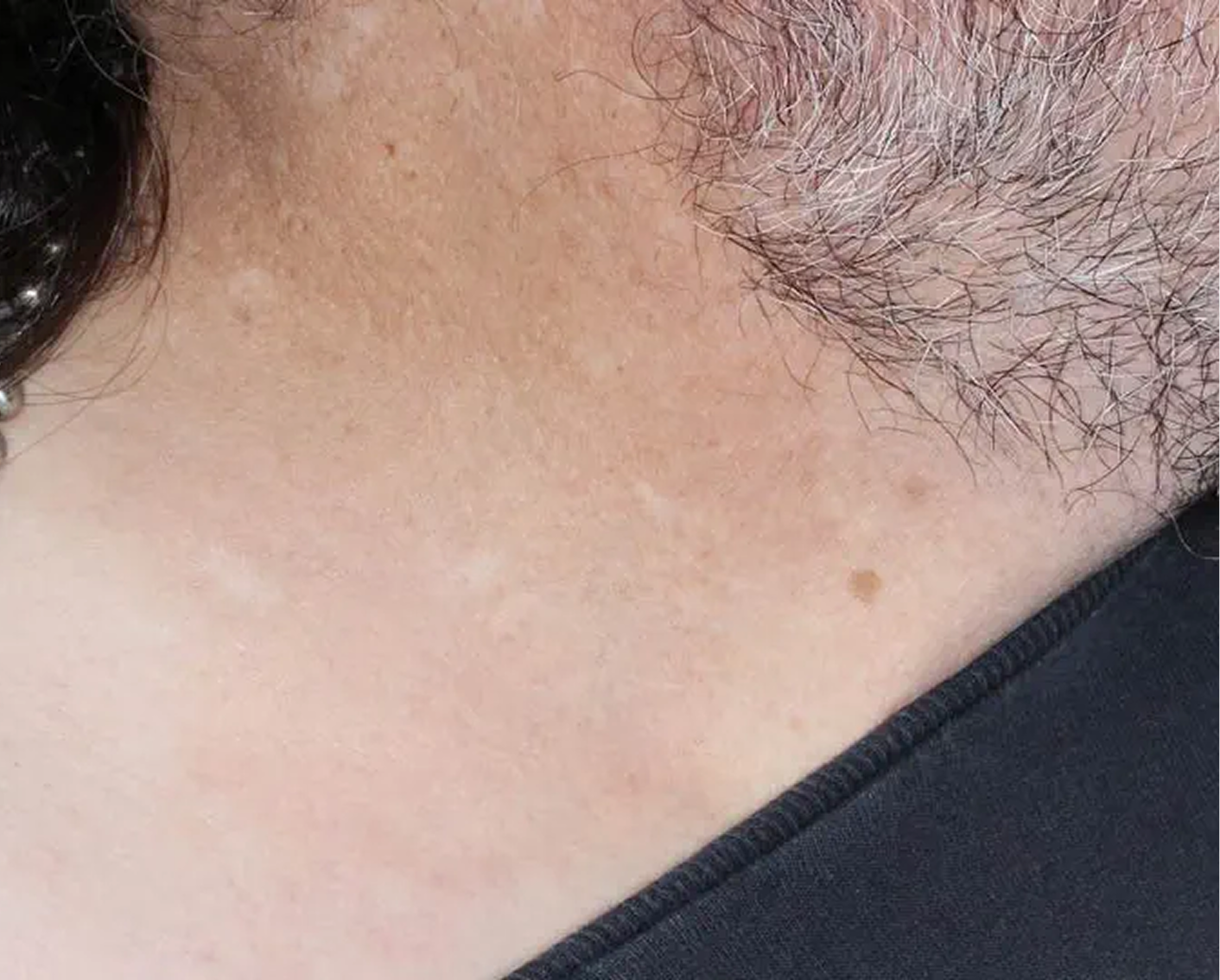
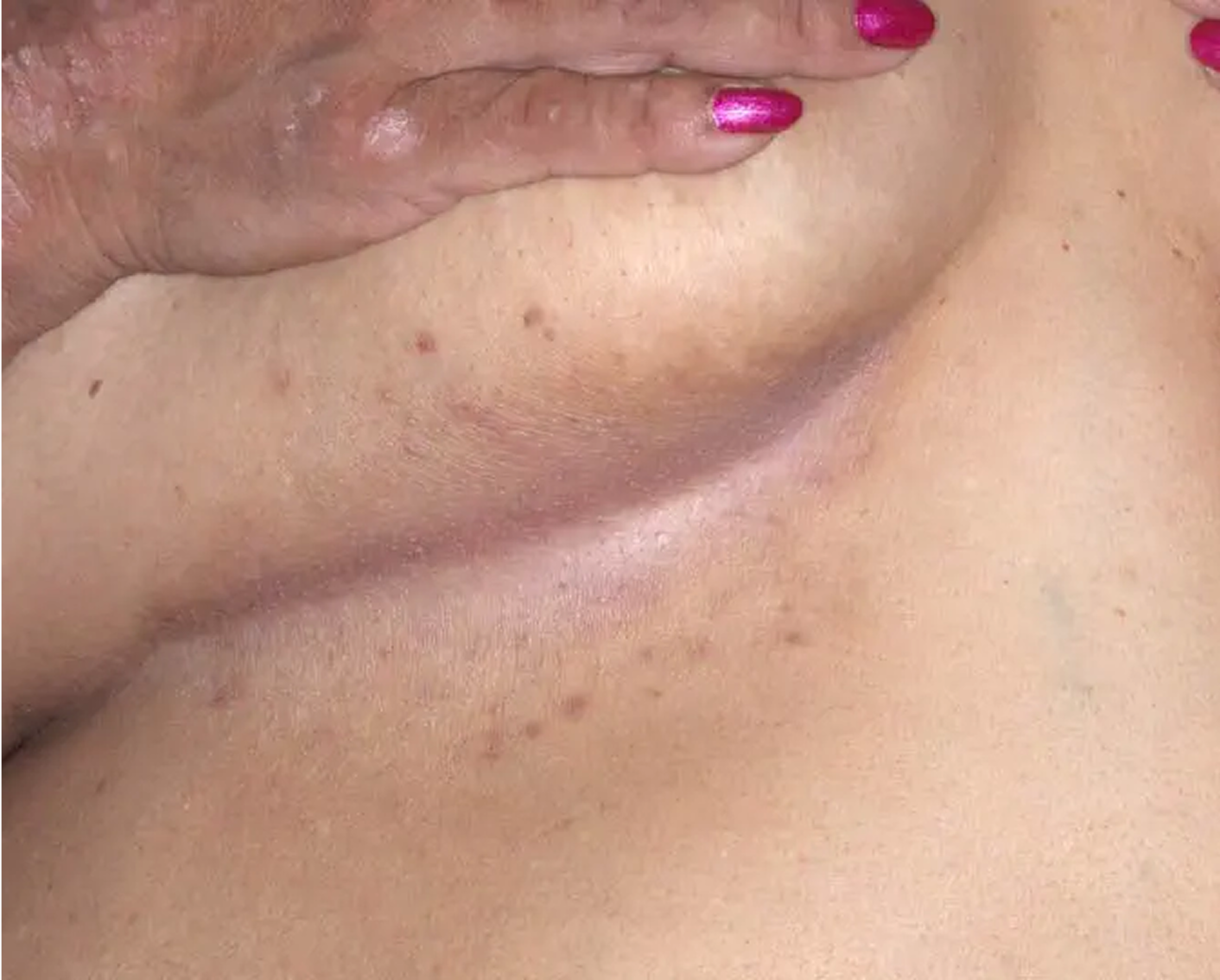
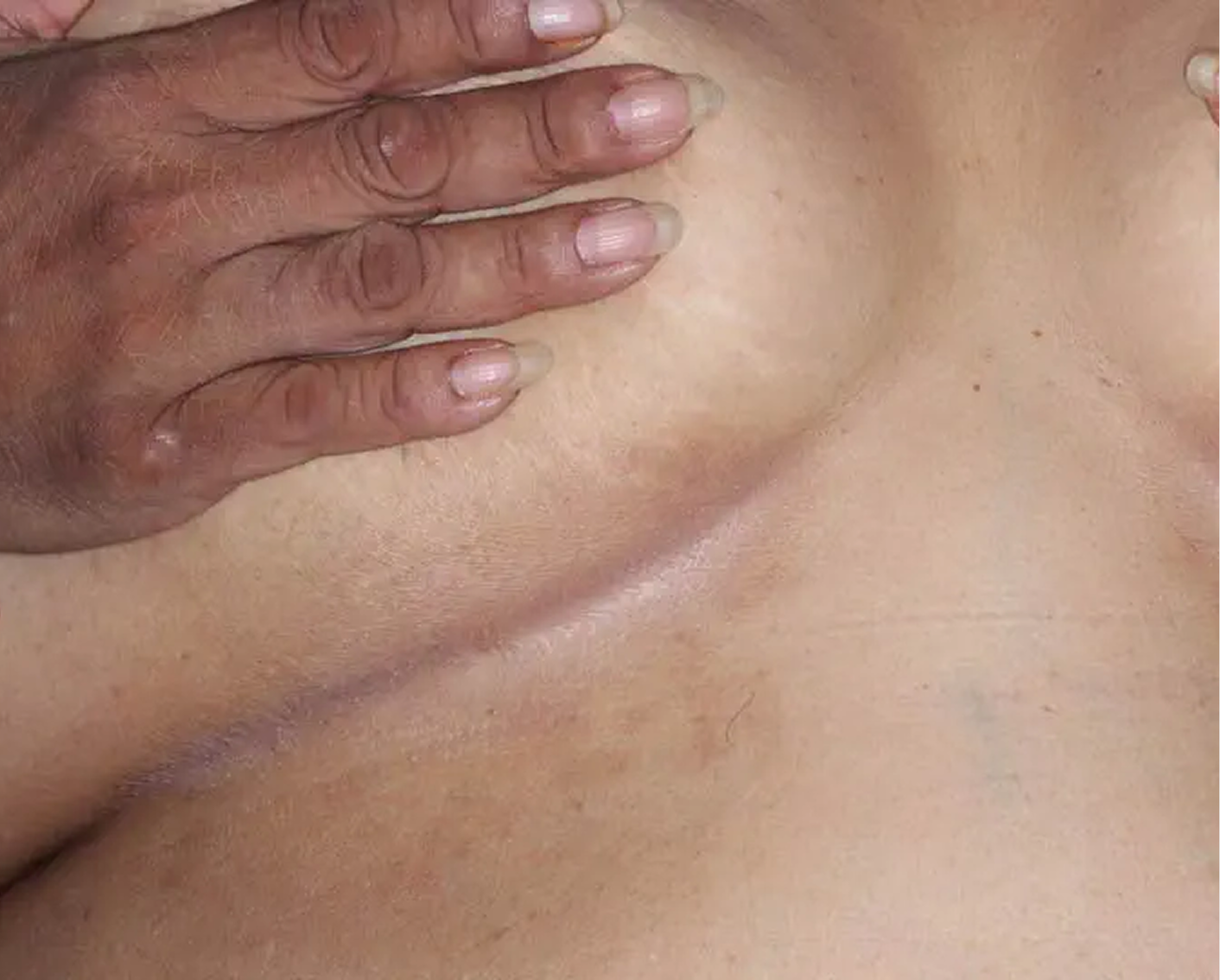
Axilla3
AGE 60, MALE
Actual clinical trial patient
Baseline: I-IGA = 3
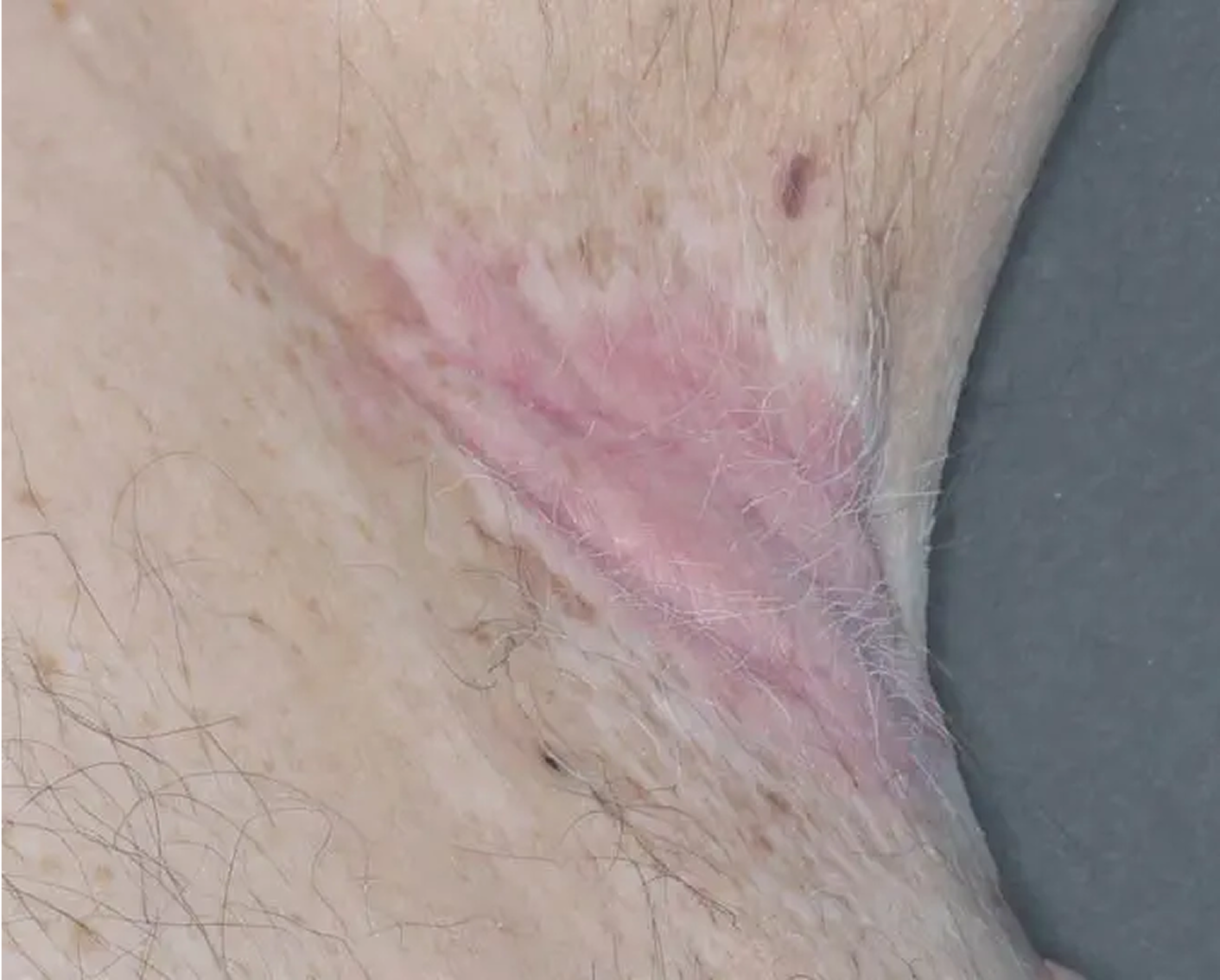
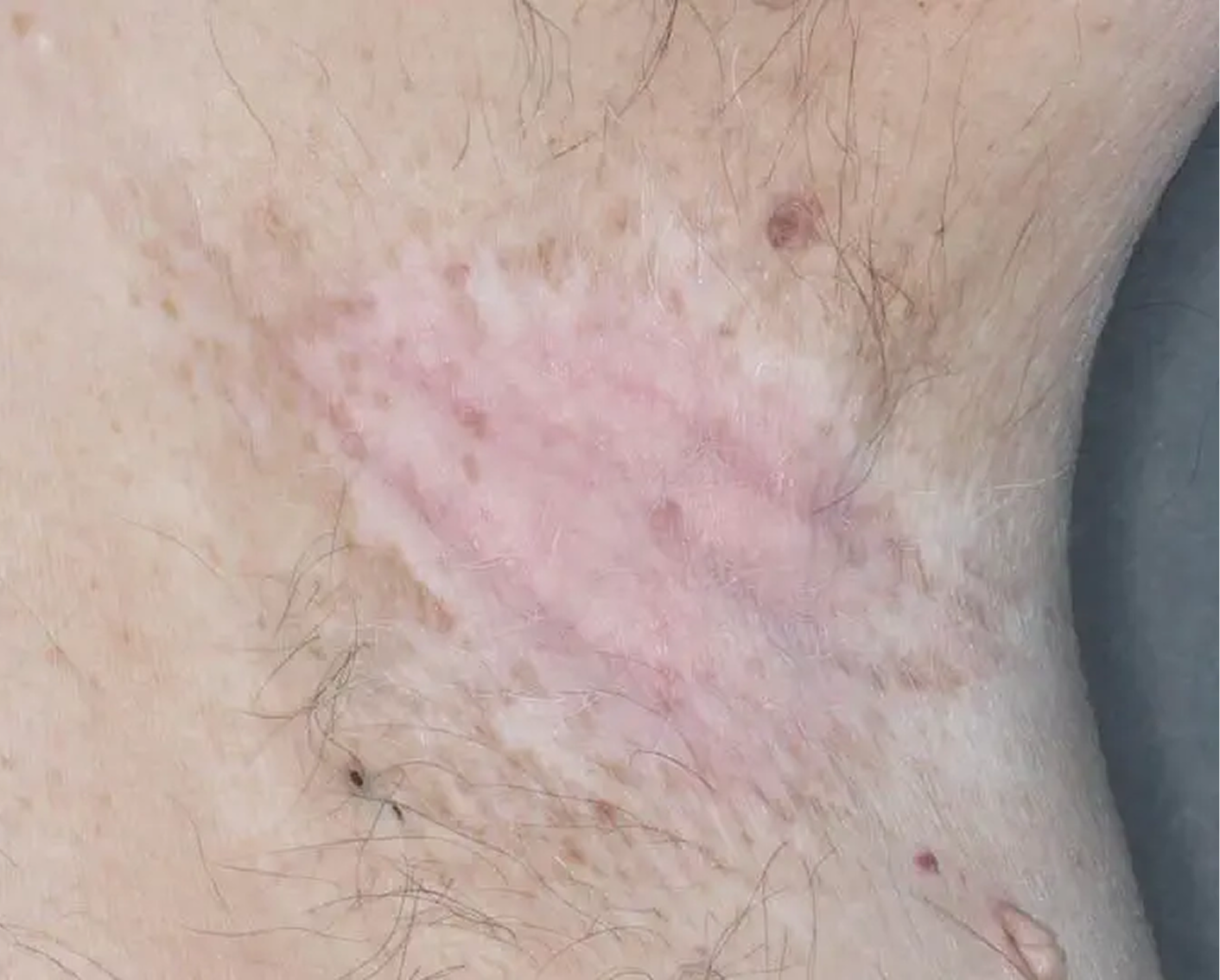
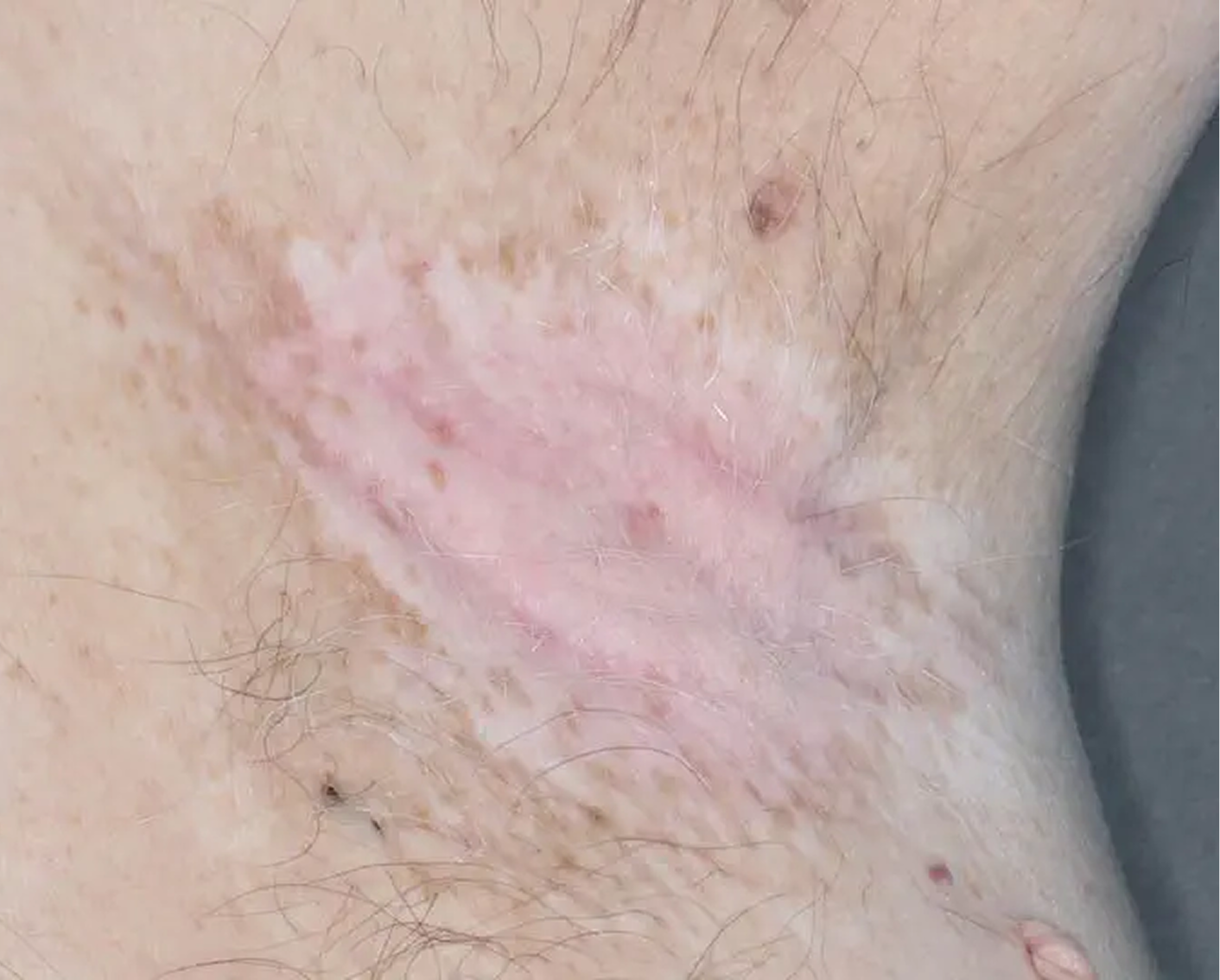
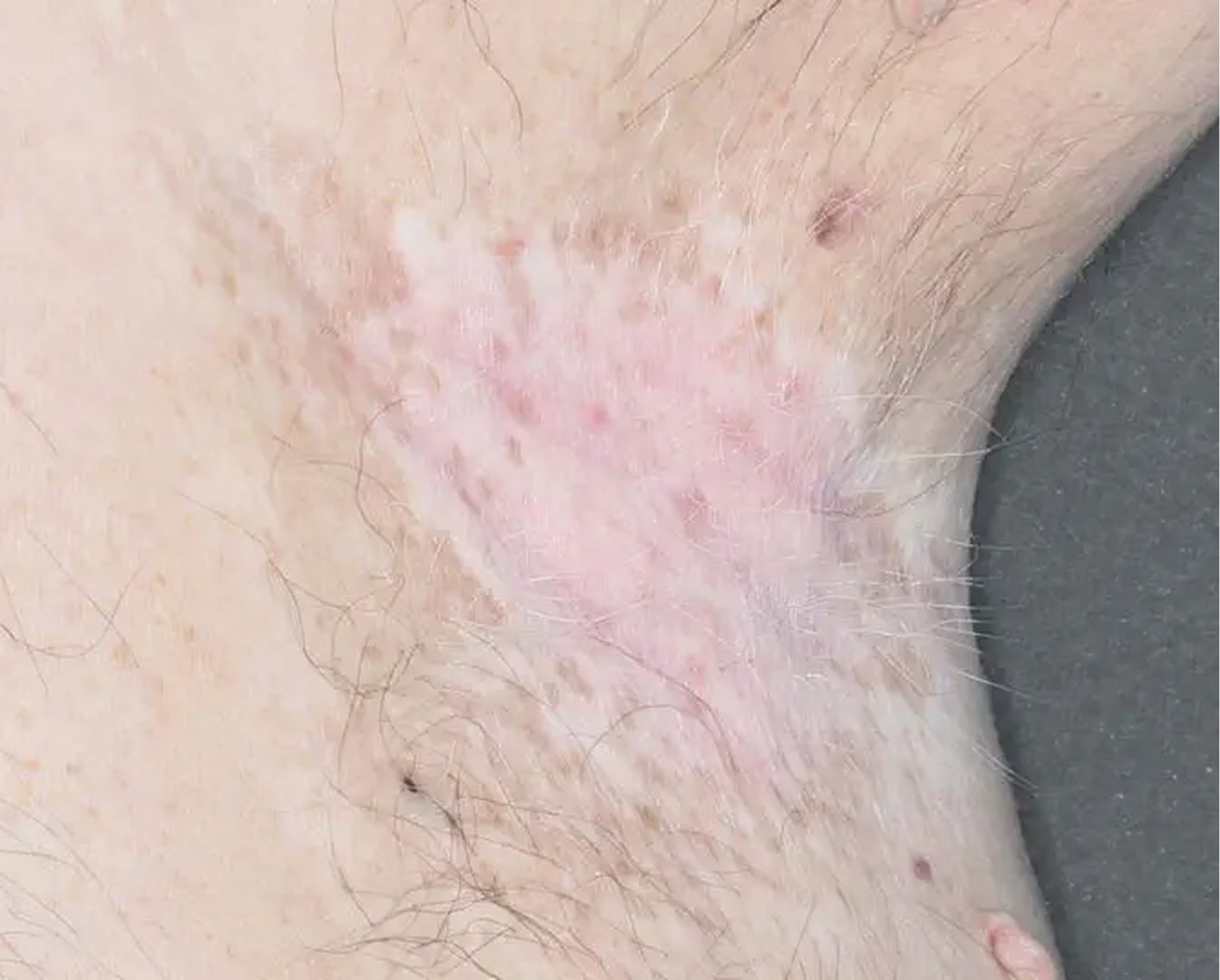
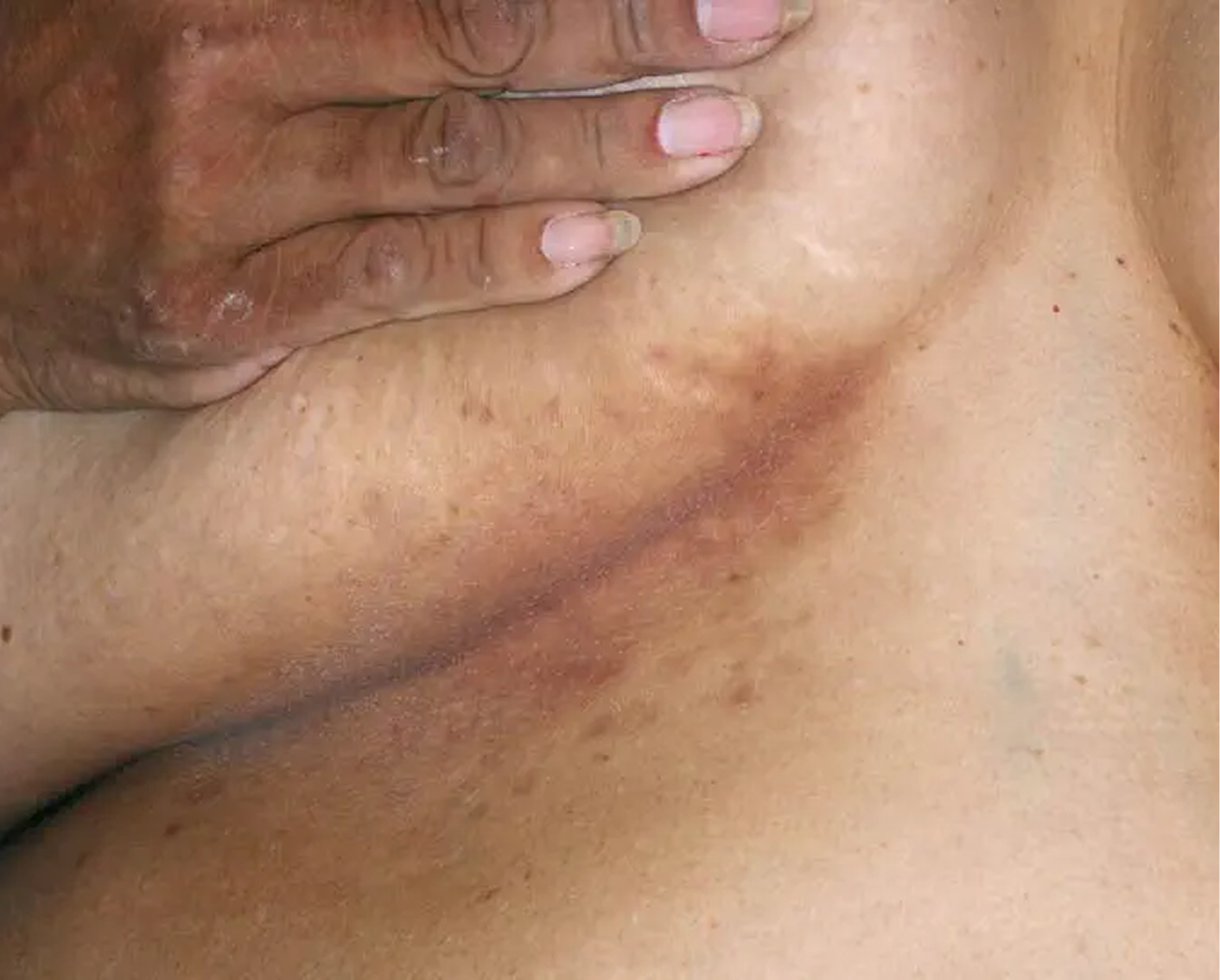
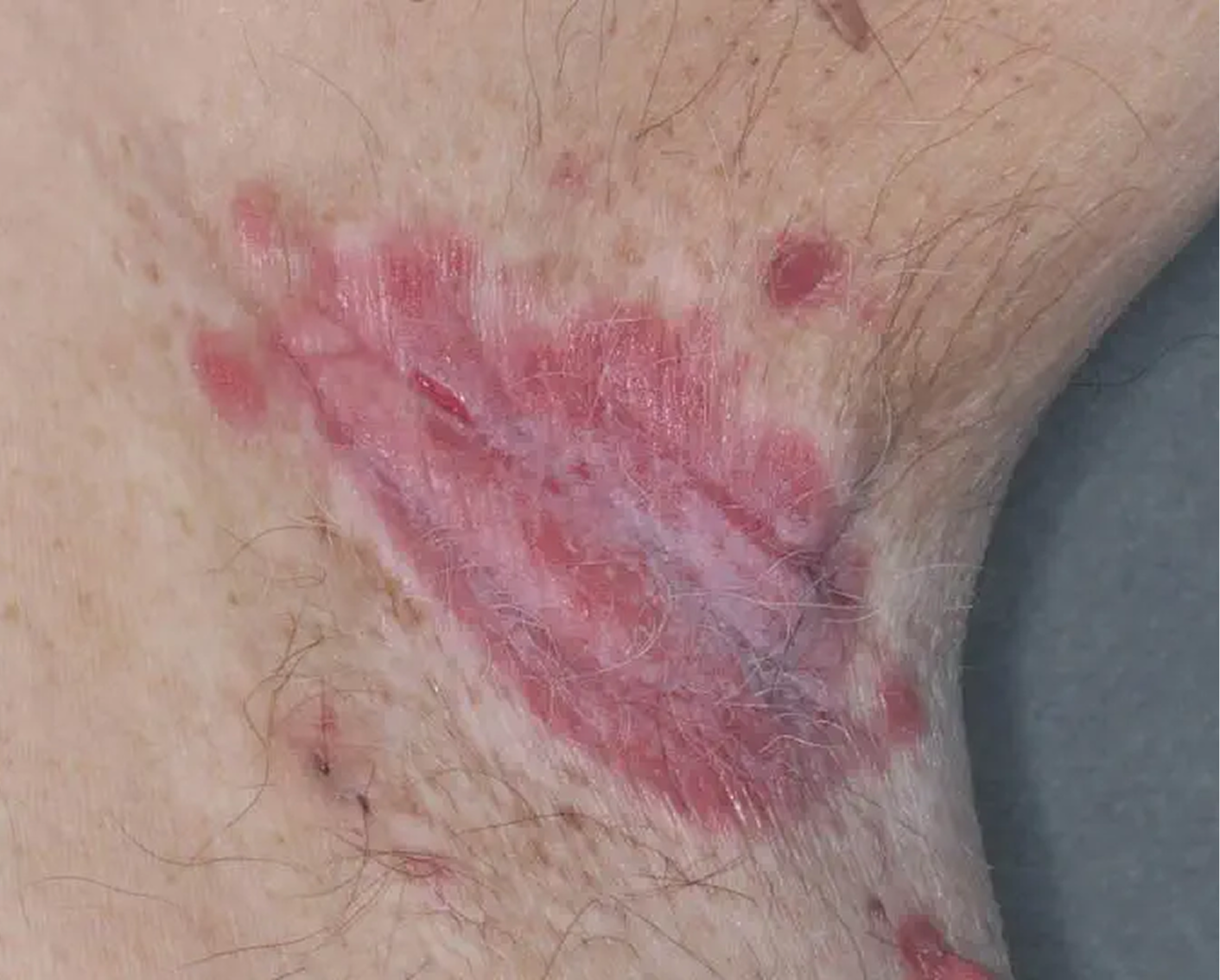
AGE 37, MALE
Actual clinical trial patient
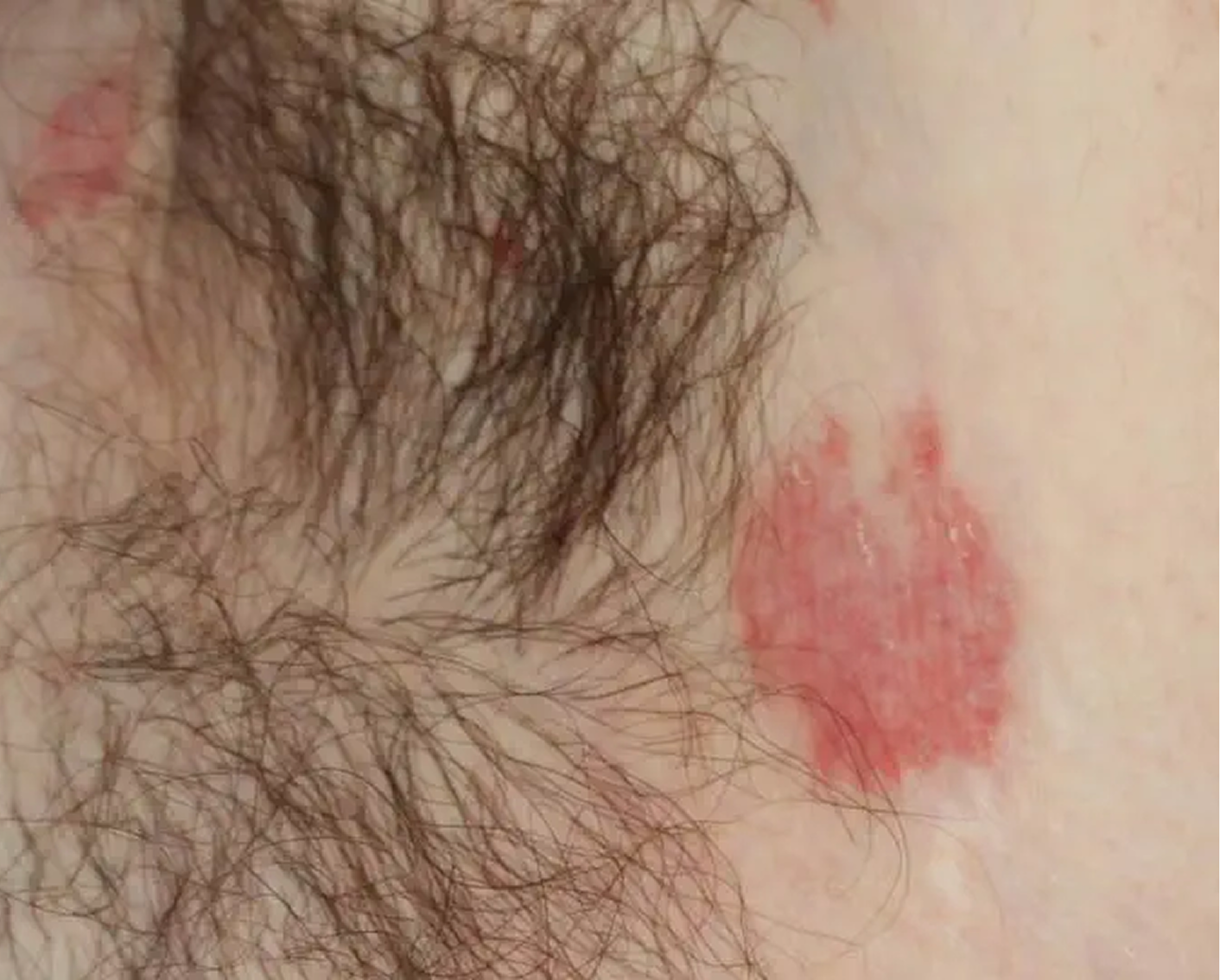
Baseline: I-IGA = 3
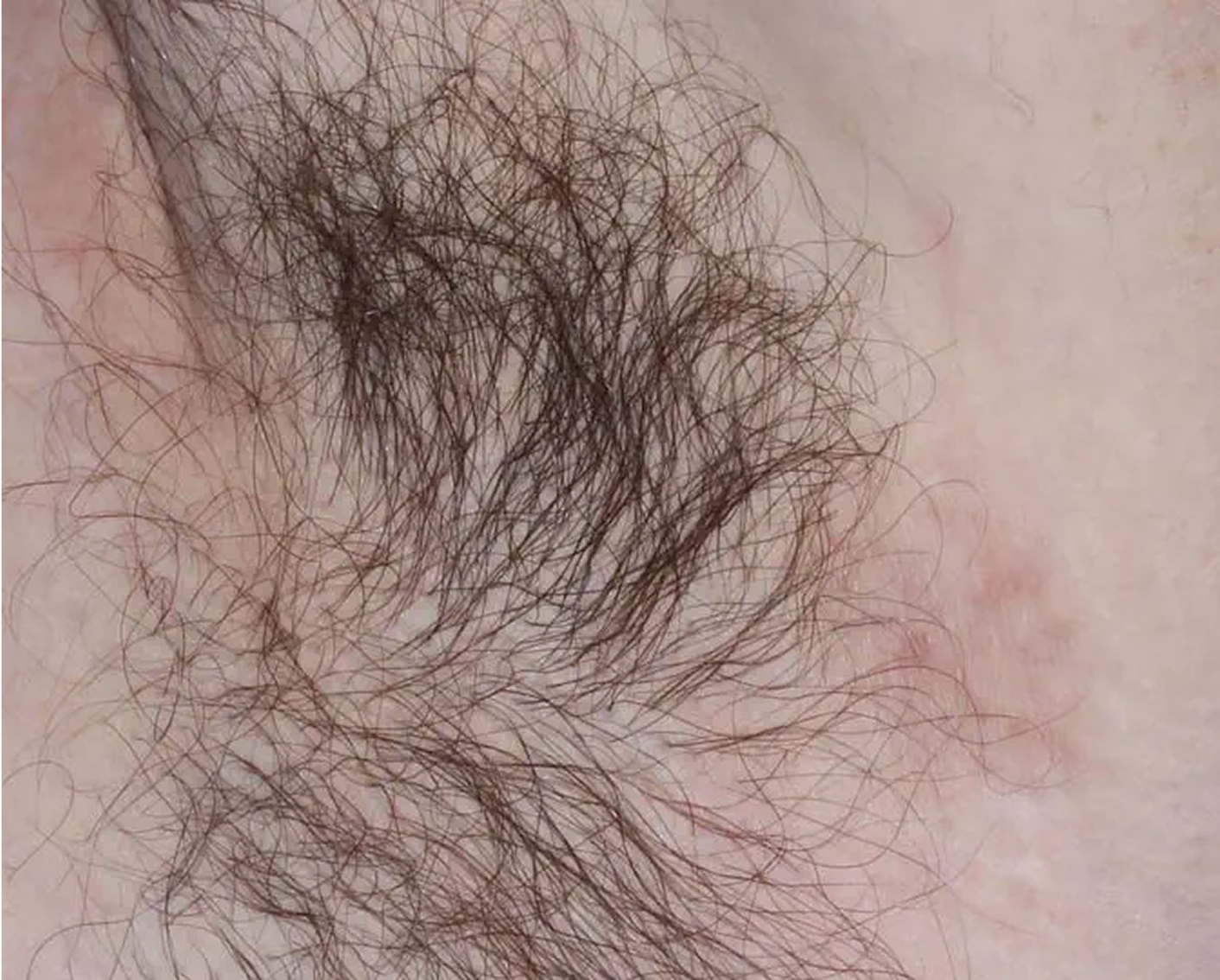
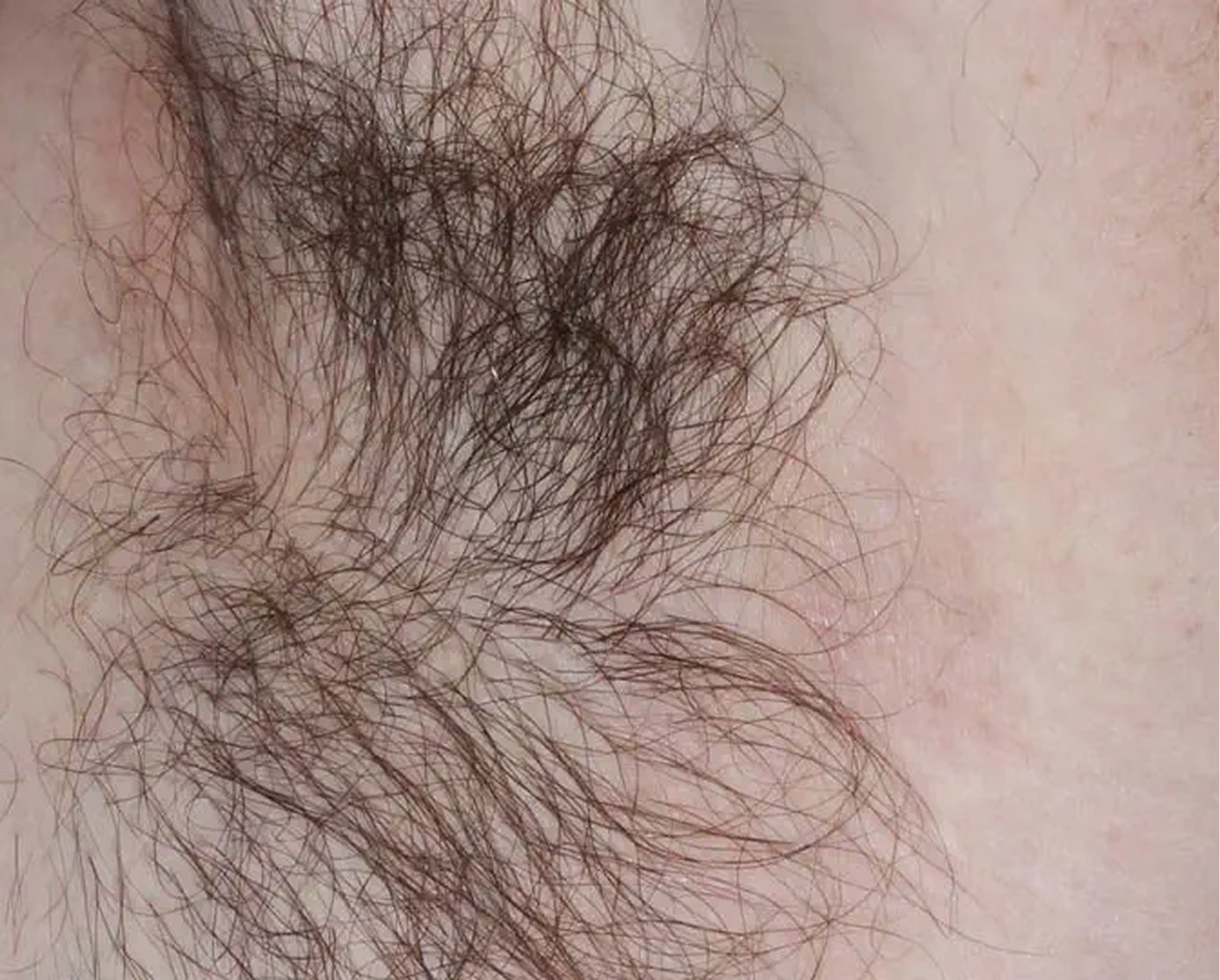
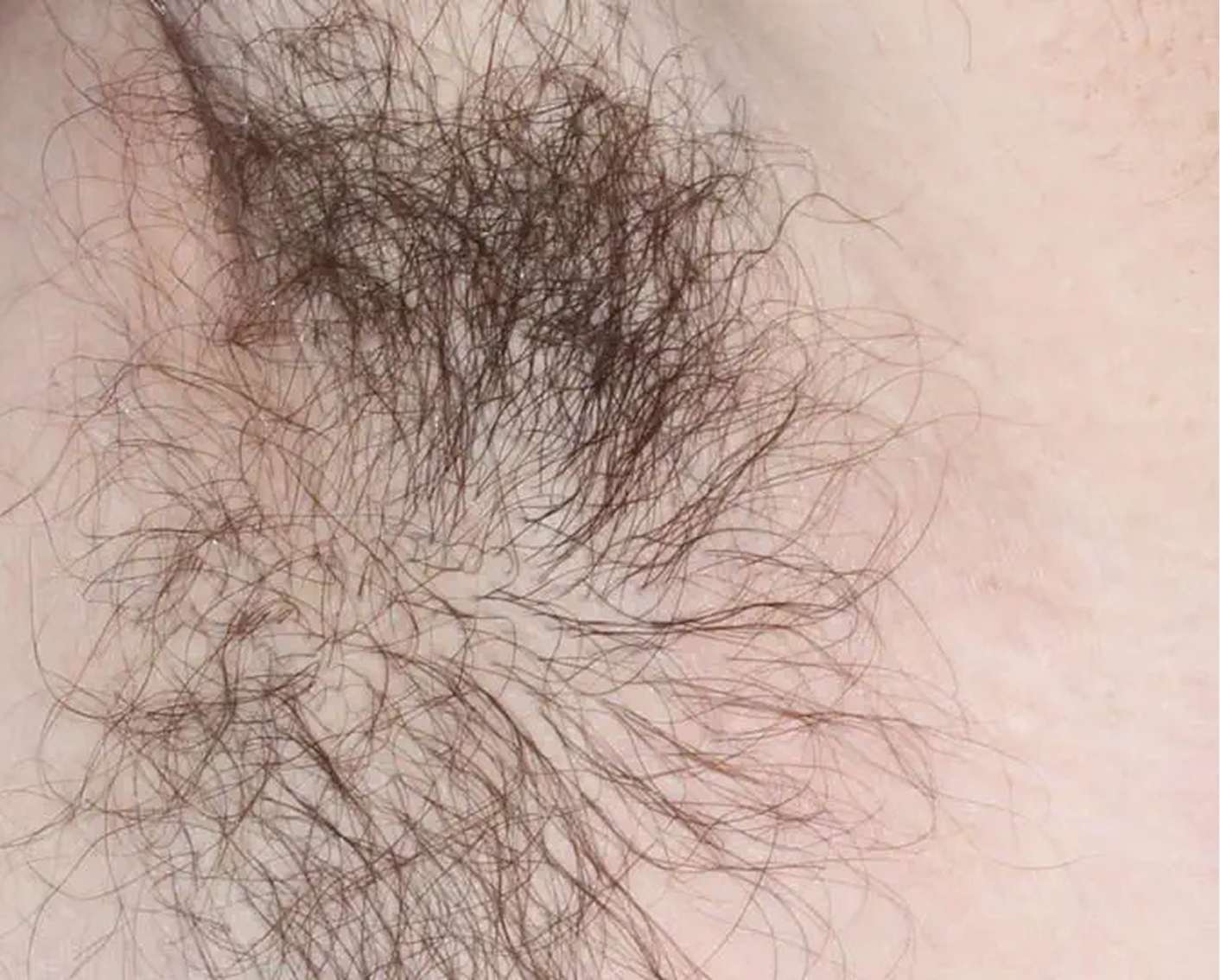
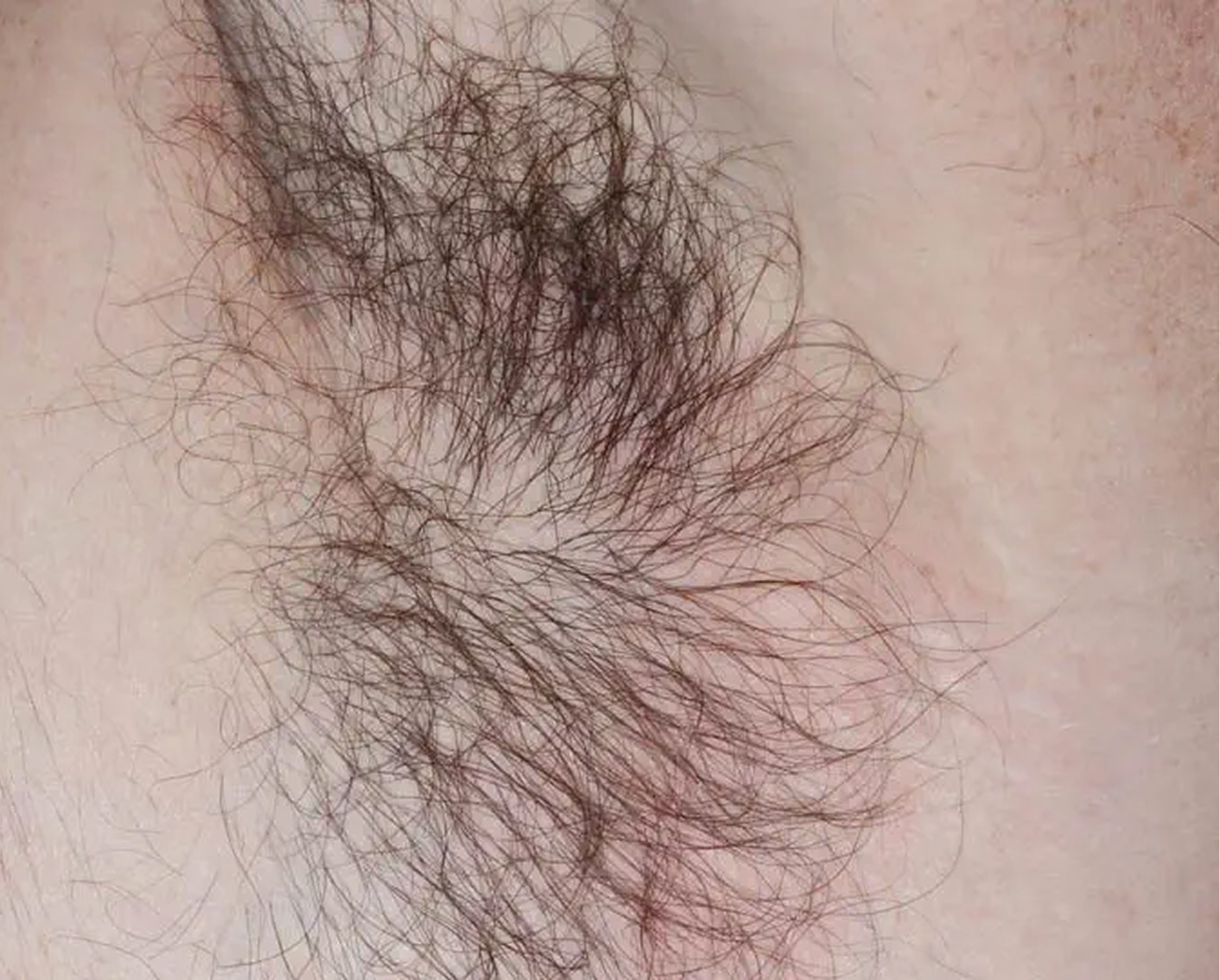
ZORYVE is the only branded topical for plaque psoriasis specifically
indicated for use in the intertriginous areas2,3
Meaningful clearance at Week 8
These patients met 2-grade improvement on IGA score but did not achieve Clear (0) or Almost Clear (1) at Week 83
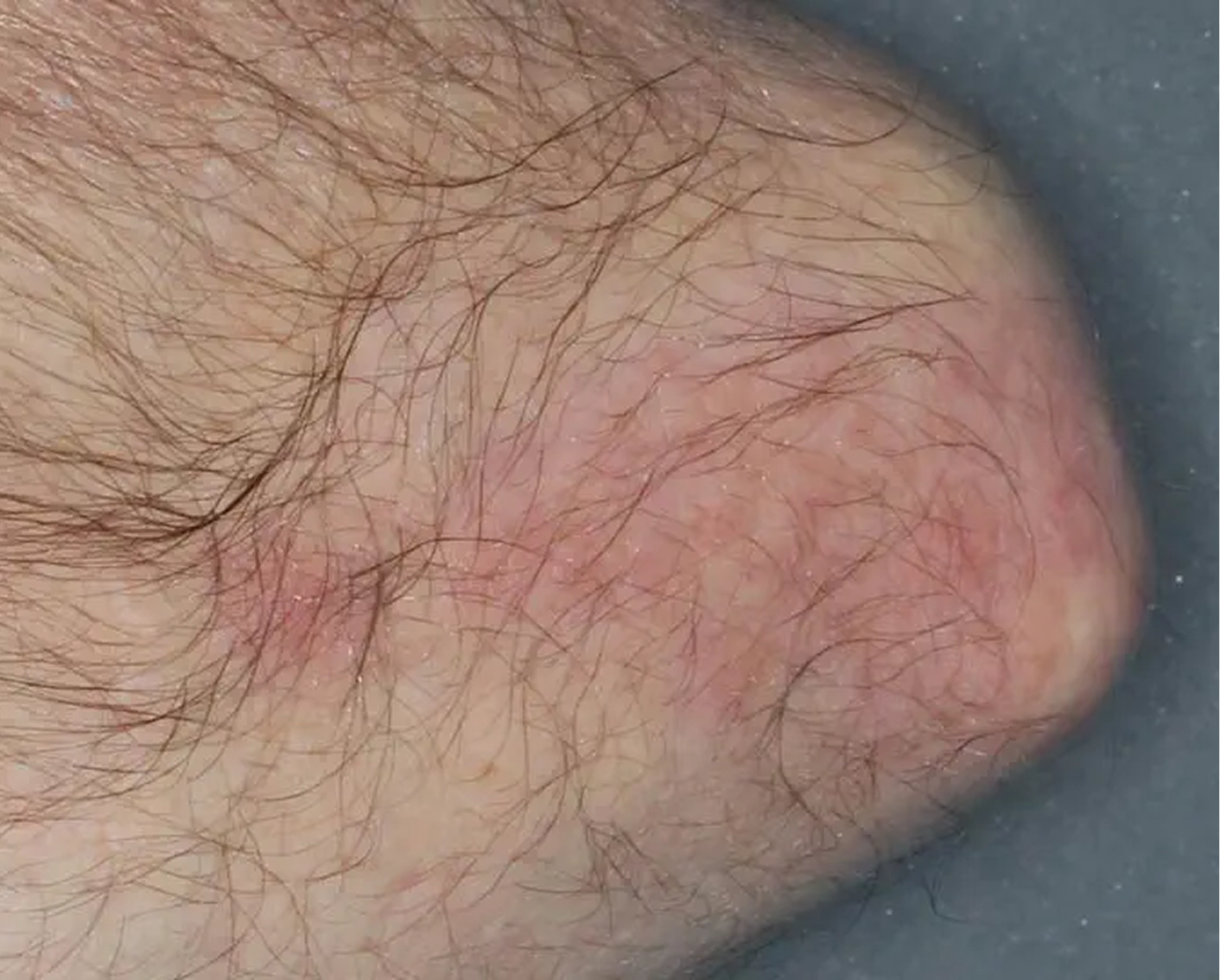
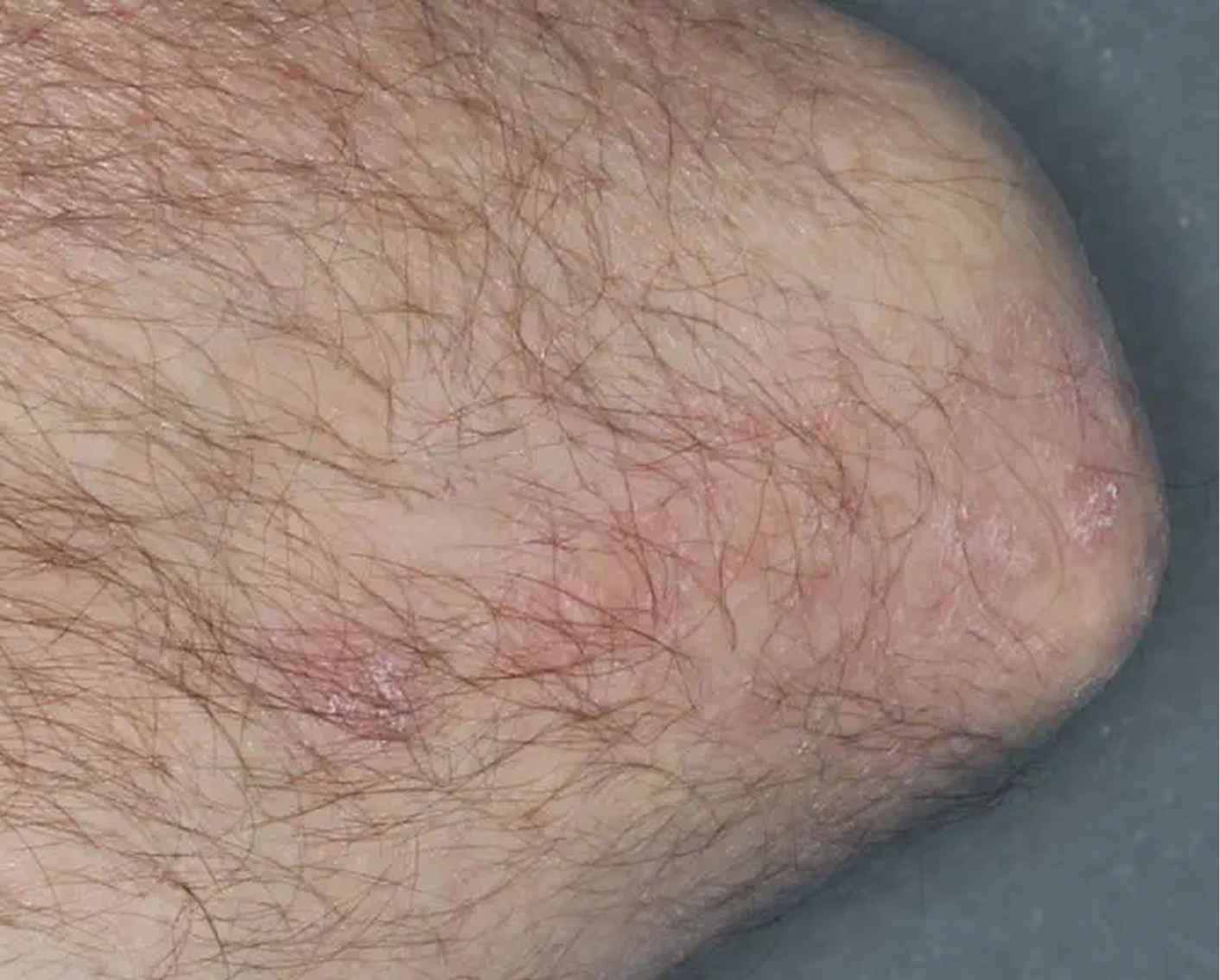
Knee3
AGE 60, MALE
Actual clinical trial patient
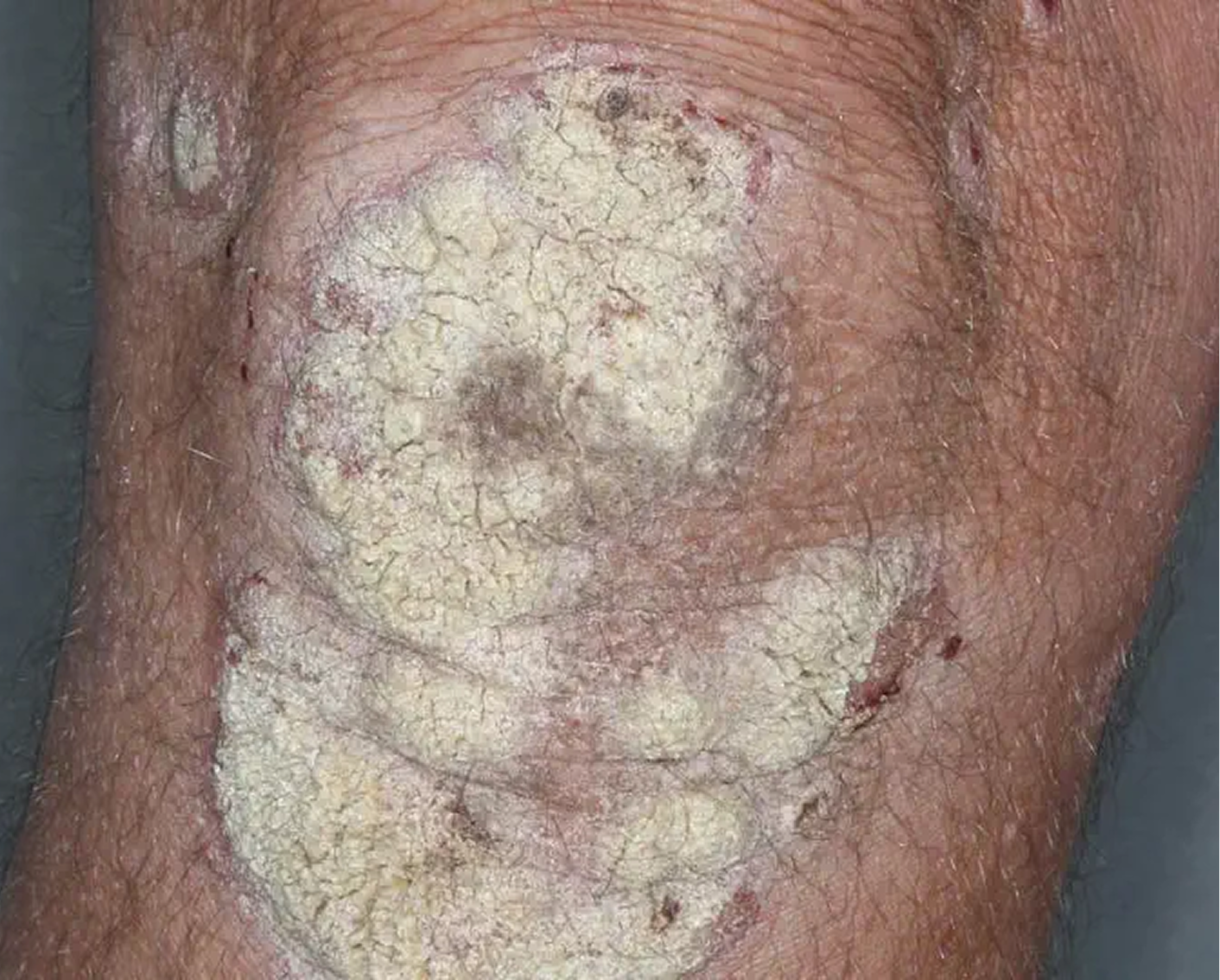
Baseline: IGA = 4
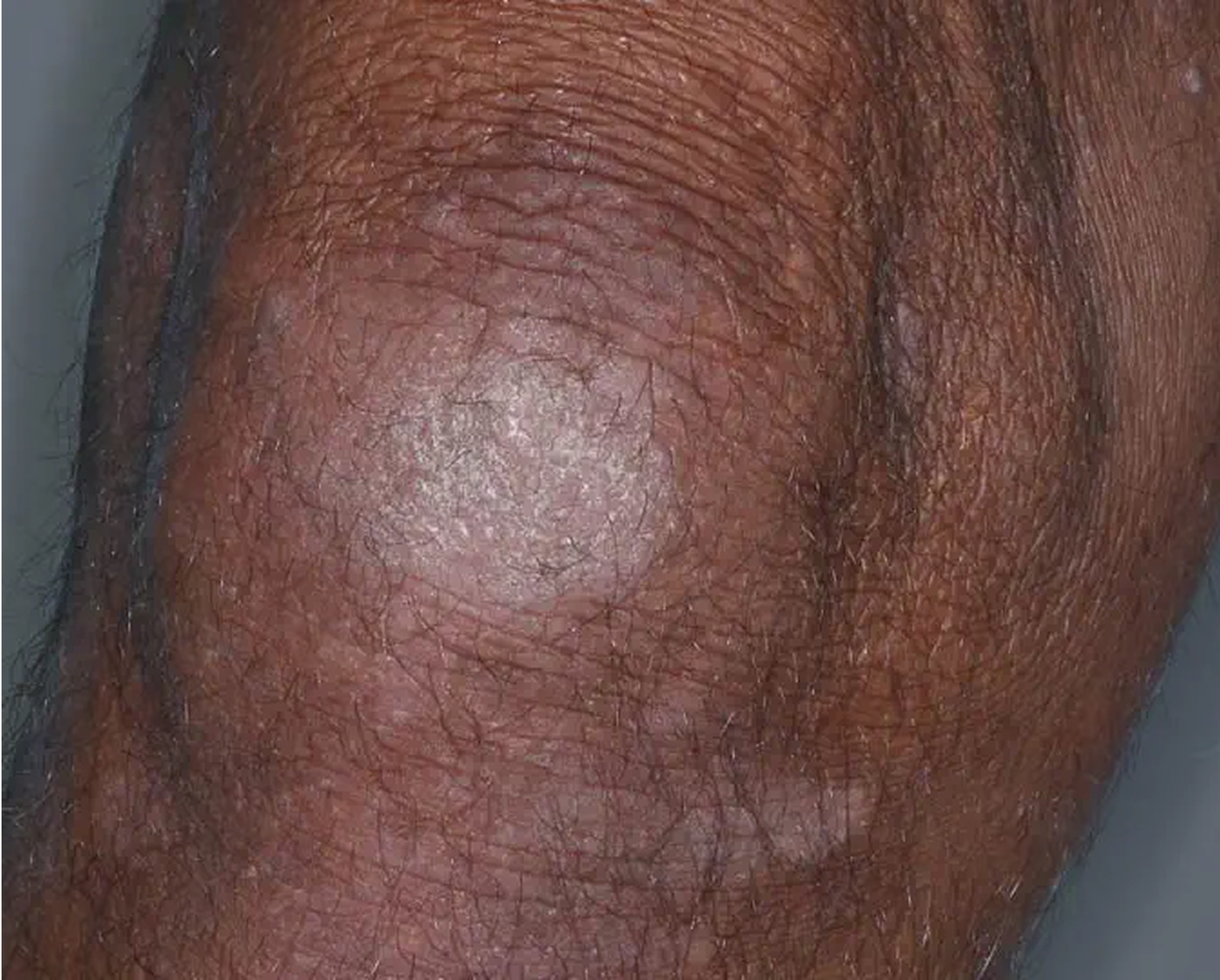
Week 8: IGA = 2
Hand3
AGE 50, MALE
Actual clinical trial patient
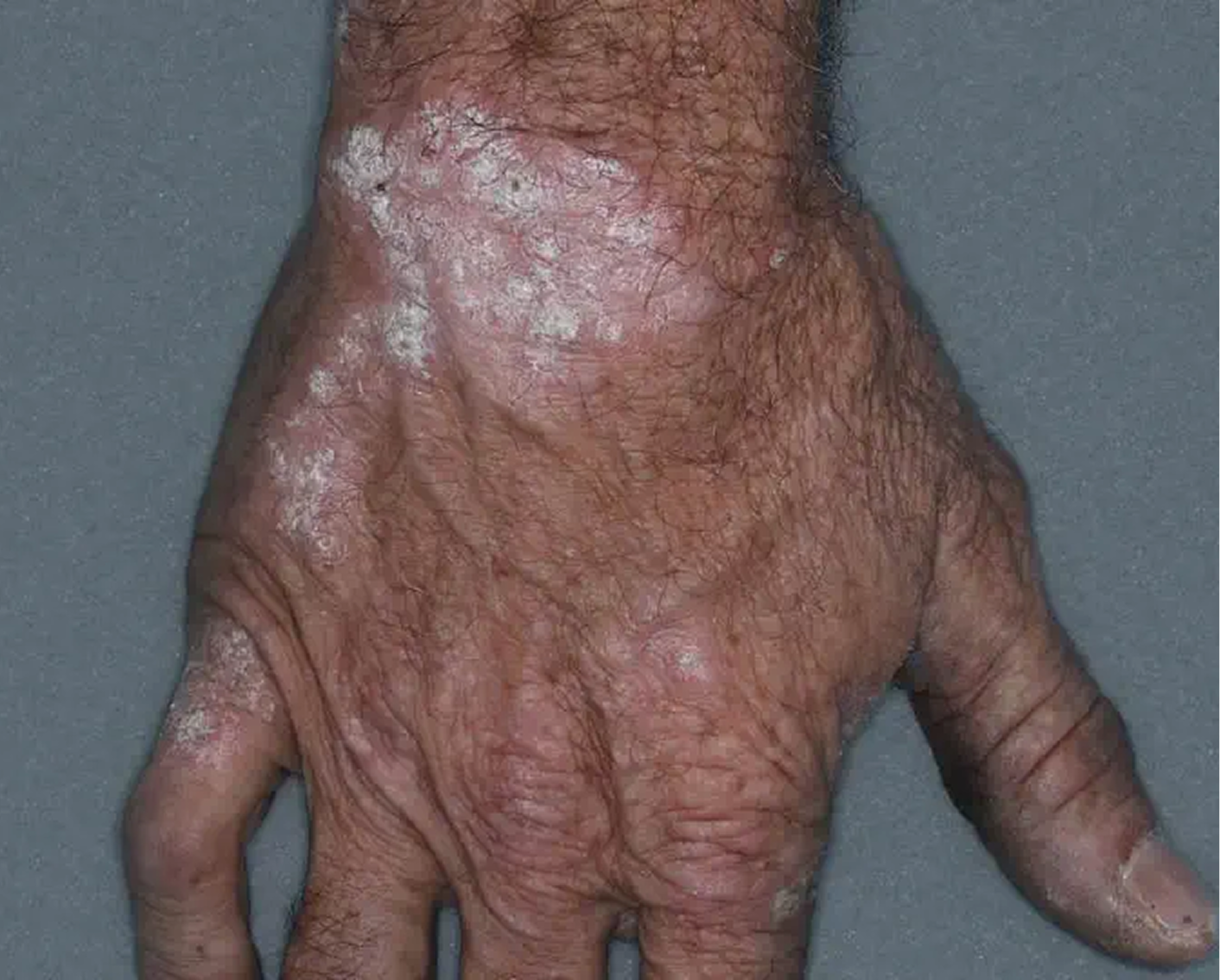
Baseline: IGA = 4
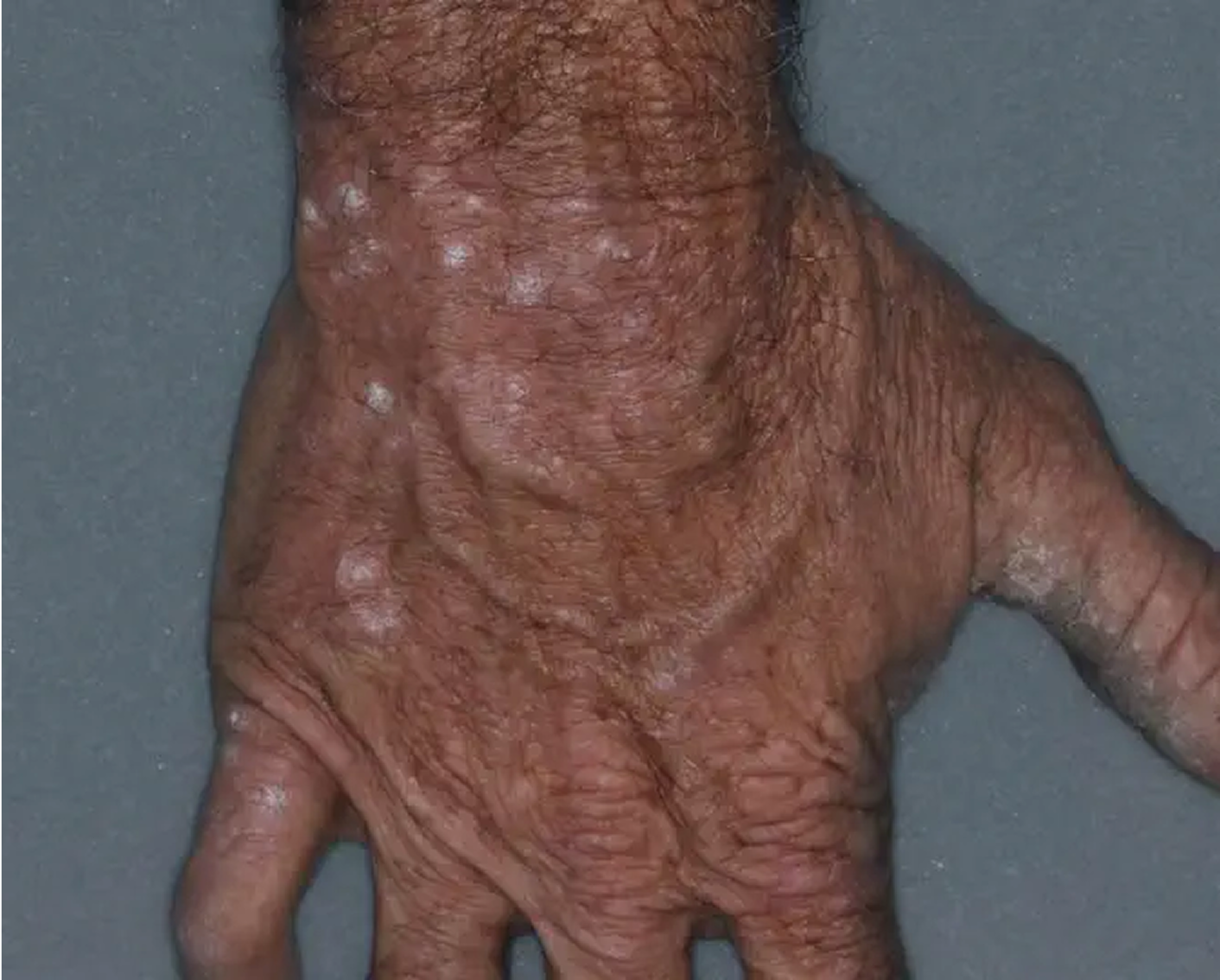
Week 8: IGA = 2
IGA Scale: 4 = Severe, 3 = Moderate,
2 = Mild, 1 = Almost Clear, 0 = Clear.
B-IGA Scale: 4 = Severe, 3 = Moderate,
2 = Mild, 1 = Almost Clear, 0 = Clear.


In a 2021 survey of >500 plaque psoriasis patients, more than 4 OUT OF 5 TOPICAL USERS (81%) wished they had more topical treatment alternatives to steroids.3*
*This survey was conducted online by The Harris Poll on behalf of Arcutis Biotherapeutics among US adults 18+ who have been diagnosed with psoriasis by a healthcare provider. The survey was conducted in 2021, among 507 plaque psoriasis patients. Figures were weighted where necessary to bring the data into line with actual proportions in the population using a multi-step weighting process.

