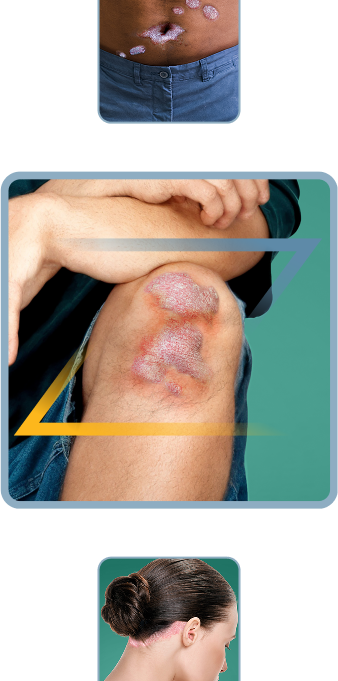
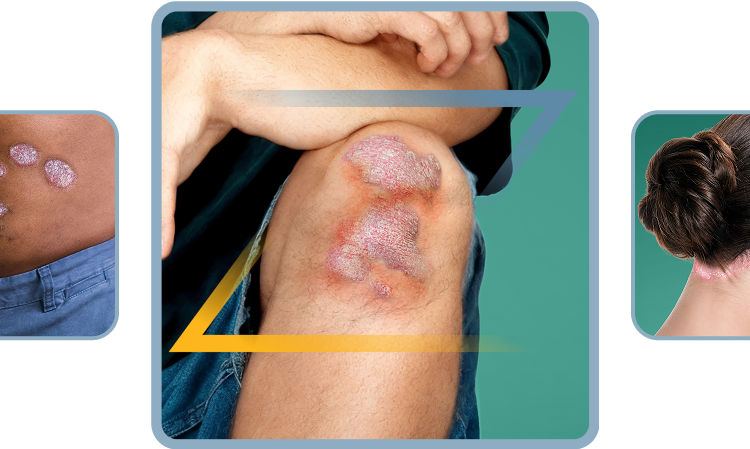
Not actual patients.
Symptoms illustrated.
Plaque Psoriasis?
ORYVE IT
RELIEVE IT
versatility to be your go-to topical.1,2


GIVE YOUR PATIENTS WITH PLAQUE PSORIASIS
THE POWER OF CHOICE
The first and only FDA-approved branded topical in foam and cream options for plaque psoriasis treatment from head to toe1-3
Simple, once-daily
treatment1,2
Simplify plaque psoriasis treatment1-3,6,7
40% of patients achieved IGA Success at Week 8 with ZORYVE cream 0.3% vs 7% with vehicle.
69% of patients achieved WI-NRS Success at Week 8 with ZORYVE cream 0.3% vs 31% with vehicle.
63% of patients achieved S-IGA Success at Week 8 with ZORYVE foam vs 21% with vehicle; 42% of patients achieved B-IGA Success at Week 8 with ZORYVE foam vs 15% with vehicle.
In ARRECTOR, 65% of patients achieved SI-NRS Success at Week 8 with ZORYVE foam vs 30% with vehicle, 63% of patients experienced WI-NRS Success at Week 8 with ZORYVE foam vs 30% with vehicle.


A 2021 survey of >500 plaque psoriasis patients showed 9 OUT OF 10 TOPICAL TREATMENT USERS (89%) were interested in trying a new topical3*
*In a survey conducted online in 2021 by the Harris Poll on behalf of Arcutis Biotherapeutics among 507 US adults who had been diagnosed with plaque psoriasis, 89% strongly or somewhat agreed that they were interested in trying a new topical treatment and 81% strongly or somewhat agreed that they wished they had more topical treatment alternatives to steroids.
One ZORYVE Direct Savings Program helps eligible, commercially insured patients get access and start ZORYVE treatment quickly and easily†‡
Patient Access Support
Savings Program
Adherence Support
Learn About Patient
Access Support
†Prescriptions will be delivered to the patient 1–2 days after processing.
‡Subject to eligibility criteria and maximum program limitation. This offer is not valid for patients without commercial drug insurance or whose prescription claims are eligible to be reimbursed, in whole or in part, by any government program. Please see Terms and Conditions.

