
Atopic Dermatitis?
STUDIED IN A PATIENT POPULATION THAT LOOKS LIKE YOUR PRACTICE
Study Design
Study Design
One cream. Once a day. Anywhere.1
INTEGUMENT-1 and INTEGUMENT-2 pivotal study designs1:
- Two Phase 3, multicenter, randomized, double-blind, vehicle-controlled studies (INTEGUMENT-1 and -2)
- 1337 participants with mild to moderate atopic dermatitis ages 6 years and older
- ZORYVE = 884, vehicle = 453
- Once daily for 4 weeks
- Mild to moderate atopic
dermatitis (vIGA-AD = 2 or 3)2 - ≥6 years old2
- EASI score ≥52
No concomitant therapies or other moisturizers/
emollients were allowed on treated areas3
Primary endpoint
vIGA-AD Success at Week 4
Achievement of vIGA-AD score of Clear (0) or Almost Clear (1) and ≥2-grade improvement from baseline1
Key secondary endpoints
WI-NRS Success at Weeks 1, 2, and 4
Achievement of a ≥4-point improvement from baseline for patients ≥12 years with a baseline score of ≥41
EASI-75 at Week 4
Achievement of a 75% reduction in EASI score from baseline2
vIGA-AD score of Clear or Almost Clear at Weeks 1, 2, and 4
Achievement of a vIGA-AD of 0 or 12
Exploratory endpoint
Daily WI-NRS
Change from baseline in daily WI-NRS4
Studied in a diverse patient population with mild to moderate atopic dermatitis1,2
- Up to 88% BSA (range: 3%–88%; mean: 14%)1
- Age range: 6–91 years old (mean: 28 years)1,2
- Evaluated across race, ethnicities, and skin types (54% lighter skin; 46% darker skin)2*
*60% White; 40% non-White (20% African American, 13% Asian); 54% Fitzpatrick I-III, 46% Fitzpatrick IV-VI.
Clearance Rates
CLEARER SKIN AS EARLY AS WEEK 1 FOR PATIENTS AGED 6+
Primary endpoint
vIGA-AD Success at Week 4
Secondary endpoint
vIGA-AD Clear/Almost Clear at Week 4
2x as many patients achieved vIGA-AD Success at Week 4 with ZORYVE4
Data are pooled analyses of the INTEGUMENT-1 and -2 studies.
More patients achieved vIGA-AD Success
as early as Week 12,3†
Data are pooled analyses of the INTEGUMENT-1 and -2 studies.
Nearly 2x as many patients had Clear or Almost Clear skin at Week 4 with ZORYVE4
Data are pooled analyses of the INTEGUMENT-1 and -2 studies
Some patients had Clear/Almost Clear skin
as early as Week 13,4†
Data are pooled analyses of the INTEGUMENT-1 and -2 studies
BASELINE
vIGA-AD = 3
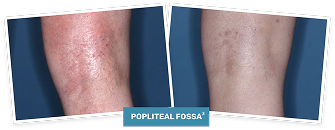
WEEK 4
vIGA-AD = 1
Actual clinical trial patient
9 in 10 patients aged 6+ experienced improvements
in clinical signs at Week 43,5
Pooled EASI response rates, EASI-75 was a secondary endpoint. Analysis provides a summary of EASI improvement for every patient in INTEGUMENT-1 and INTEGUMENT-2. EASI response rates are defined as percentage of patients with improved EASI scores from baseline to Week 4. EASI scores evaluate the following atopic dermatitis symptoms: erythema, skin thickness (induration, papulation, swelling), excoriations, lichenification, and percentage of region affected. EASI-50 = 50% reduction in EASI score from baseline. EASI-75 = 75% reduction in EASI score from baseline. EASI-90 = 90% reduction in EASI score from baseline. EASI-100 = 100% reduction reduction in EASI scores from baseline. These efficacy data are not in the Prescribing Information for ZORYVE.3
Itch Relief
ITCH IMPROVEMENT AT WEEK 4 WITH RESULTS OBSERVED WITHIN 24 HOURS FOR PATIENTS AGED 6+
Secondary endpoint
Nearly2x
as many patients achieved WI-NRS Success at Week 4 with ZORYVE (32%; n = 542) vs with vehicle (17%; n = 271)4
Daily WI-NRS2
WI-NRS Success=Achievement of ≥4-point improvement for patients ≥12 years with a baseline score of ≥4.
Data are pooled analyses of the INTEGUMENT-1 and -2 studies.
#An exploratory endpoint in INTEGUMENT-1 and INTEGUMENT-2 was mean change from baseline in daily WI-NRS after first application with ZORYVE (n=884) vs vehicle (n=453). Evaluated in all patients, not just those with baseline WI-NRS ≥4. These data are not included in the Prescribing Information.
Greater itch improvement experienced within 24 HOURS after first application with ZORYVE vs vehicle4#
WI-NRS Success=Achievement of ≥4-point improvement for patients ≥12 years with a baseline score of ≥4.
Data are pooled analyses of the INTEGUMENT-1 and -2 studies.
#An exploratory endpoint in INTEGUMENT-1 and INTEGUMENT-2 was mean change from baseline in daily WI-NRS after first application with ZORYVE (n=884) vs vehicle (n=453). Evaluated in all patients, not just those with baseline WI-NRS ≥4. These data are not included in the Prescribing Information.
BASELINE
vIGA-AD = 3
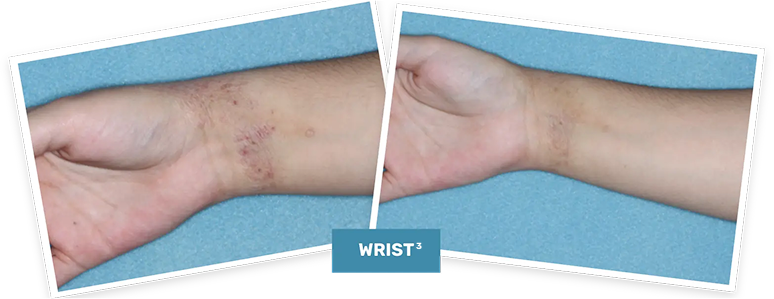
WEEK 4
vIGA-AD = 0
Actual clinical trial patient

