
Atopic Dermatitis?
STUDIED IN A PATIENT POPULATION THAT LOOKS LIKE YOUR PRACTICE
Study Design
Study Design
One cream. Once a day. Anywhere.1
INTEGUMENT-PED study design1:
- One multicenter, randomized, double-blind, vehicle-controlled study (INTEGUMENT-PED)
- 652 participants with mild to moderate atopic dermatitis ages 2 to 5 years
- ZORYVE = 436, vehicle = 215
- Once daily for 4 weeks
- Mild to moderate atopic
dermatitis (vIGA-AD = 2 or 3)6 - 2–5 years old6
- EASI score ≥56
No concomitant therapies or other moisturizers/
emollients were allowed on treated areas6
Primary endpoint
vIGA-AD Success at Week 4
Achievement of vIGA-AD score of Clear (0) or Almost Clear (1) and ≥2-grade improvement from baseline1
Key secondary endpoints
EASI-75 at Week 4
Achievement of a 75% reduction in EASI score from baseline6
vIGA-AD score of 0 or 1 at Weeks 1, 2, and 4
Achievement of vIGA-AD 0 or 11
Exploratory endpoints
WI-NRS Success at Week 4
Achievement of a ≥4 point improvement from baseline for patients aged 2–5 with a baseline score of ≥46
Daily WI-NRS
Mean change from baseline in daily WI-NRS6
Studied in a diverse patient population with mild to moderate atopic dermatitis1,6
- Up to 82% BSA (range: 3%–82%; mean: 22%)1
- Age range: 2–5 years old (mean: 3 years)1
- Evaluated across race, ethnicities, and skin types (66% lighter skin; 34% darker skin)6*
*69% White; 31% non-White (15% African American, 8% Asian); 66% Fitzpatrick I-III, 34% Fitzpatrick IV-VI.
Clearance Rates
CLEARER SKIN AS EARLY AS WEEK 1 FOR PATIENTS AGED 2–5
Primary endpoint
vIGA-AD Success at Week 4
Secondary endpoint
vIGA-AD Clear/Almost Clear at Week 4
Twice as many children aged 2–5 achieved vIGA-AD Success at Week 4 with ZORYVE1,6
Some patients achieved clearer skin
as early as Week 16†
Over 2x as many patients had Clear or
Almost Clear skin at Week 4 with ZORYVE1,6
More children achieved Clear/Almost Clear skin
as early as Week 16†
BASELINE
vIGA-AD = 3
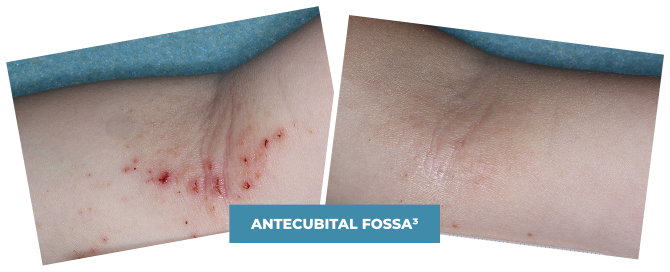
WEEK 4
vIGA-AD = 1
Actual clinical trial patient
Nearly 9 in 10 patients aged 2–5 experienced improvements
in clinical signs at Week 43
Analysis provides a summary of EASI improvement for every patient in INTEGUMENT-PED, EASI-75 was a secondary endpoint. EASI response rates are defined as percentage of patients with improved EASI scores from baseline to Week 4. EASI scores evaluate the following atopic dermatitis symptoms: erythema, skin thickness (induration, papulation, swelling), excoriations, lichenification, and percentage of region affected. EASI-50 = 50% reduction in EASI score from baseline. EASI-75 = 75% reduction in EASI score from baseline. EASI-90 = 90% reduction in EASI score from baseline. EASI-100 = 100% reduction in EASI score from baseline. These efficacy data are not in the Prescribing Information for ZORYVE.
BASELINE
vIGA-AD = 3
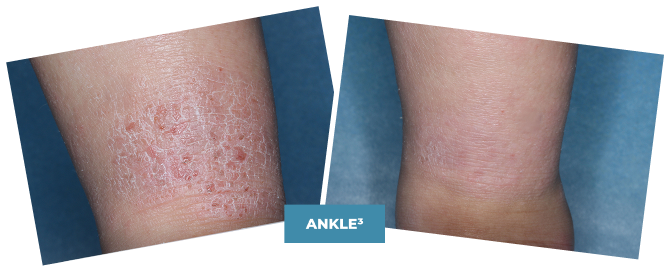
WEEK 4
vIGA-AD = 1
Actual clinical trial patient
Exploratory Endpoint - Itch Reduction
REDUCTION IN ITCH FOR PATIENTS AGED 2–56
35%
of children achieved WI-NRS Success at Week 4 with ZORYVE (n=347) vs 18% with vehicle (n=160)6‡
Daily WI-NRS6
‡Exploratory endpoints were WI-NRS Success at Week 4 and mean change from baseline (Cfb) in daily WI-NRS. WI-NRS Success was assessed by caregivers using a WI-NRS validated in patients age 12+. The difference in mean Cfb in daily WI-NRS was evaluated after first application with ZORYVE vs vehicle. These data are not included in the Prescribing Information.6
Greater itch improvement experienced within 24 HOURS after first application with ZORYVE vs vehicle6‡
‡Exploratory endpoints were WI-NRS Success at Week 4 and mean change from baseline (Cfb) in daily WI-NRS. WI-NRS Success was assessed by caregivers using a WI-NRS validated in patients age 12+. The difference in mean Cfb in daily WI-NRS was evaluated after first application with ZORYVE vs vehicle. These data are not included in the Prescribing Information.6

